ICD-10 Diagnosis Code
H18.51–Endothelial corneal dystrophy
Title
Endothelial Corneal Dystrophy
Category
Corneal Opacity And Other Disorders Of Cornea
Description
Endothelial corneal dystrophy is characterized by corneal guttata and other morphologic abnormalities of the endothelium.
Endothelial corneal dystrophy is a broad term used to describe several diseases and conditions that affect the structure and function of the corneal endothelium. The conditions are known as corneal endotheliopathies and the ICD-9-CM coding system groups most of them under the 371.57 diagnosis code.
The cornea is the main refracting surface of the eye. To refract light properly, the anterior surface of the cornea must maintain a regular shape and the entire structure must have some degree of optical transparency. To achieve structural stability and optical transparency, the anatomy of the cornea is highly specialized.
Corneal Anatomy
The layers of the cornea are optically transparent so that the entire structure can transmit visible light. When viewed in cross-section, the cornea is a basic structure of two covering layers with a central collagen stroma. This three-layer model of corneal anatomy is based on structural and biochemical differences between the layers.
The outer covering layer is called the anterior cornea. This layer is composed of the corneal epithelium and a basement membrane called Bowman’s membrane. The anterior cornea functions as a semi-permeable membrane that keeps pathogens out of the eye but allows nutrients from the pre-ocular tear film to nourish the anterior cornea.
The middle layer of the cornea is called the stroma. This layer comprises over 90% of the cornea’s thickness and is composed of tightly packed collagen fibrils in an orderly lamellar arrangement. The spacing and arrangement of the fibrils makes the stroma optically transparent.
To maintain optical transparency, the stroma must be maintained in a state of relative deturgescence and the normal level of stromal hydration is 78% water. The inability to maintain stromal deturgescenece can result in corneal edema and a loss of stromal transparency.
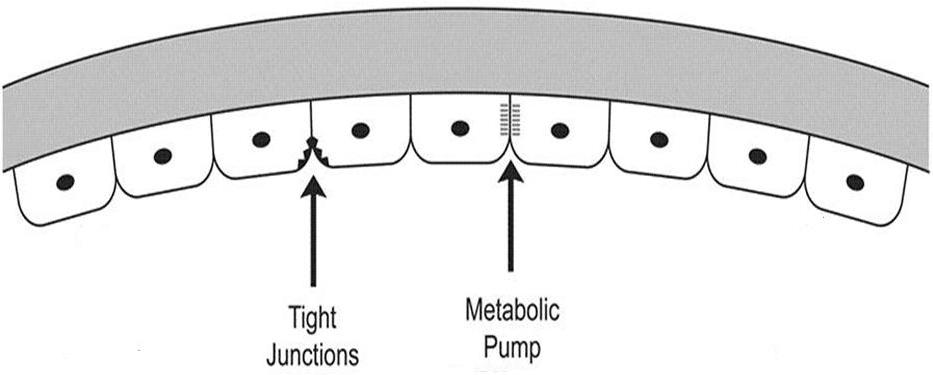
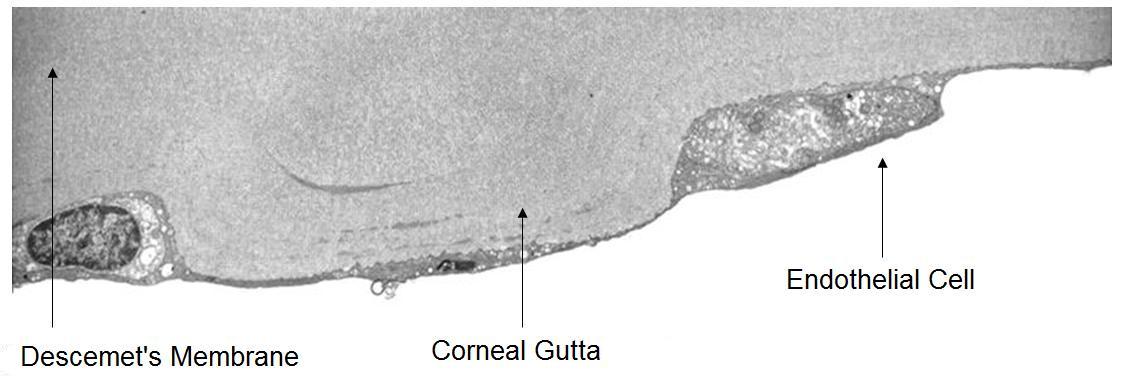
The inner covering layer is called the posterior cornea. This layer is composed of the corneal endothelium and Descemet’s membrane. The endothelium fuctions as a semi-permeable membrane that controls fluid and solute transport across the posterior surface of the cornea while Descememt’s membrane serves as a barrier against injury.
The geographic regions of the cornea differ anatomically, physiologically, immunologically and pathologically. The central region is about six millimeters in diameter while the surrounding peripheral region is approximately twelve millimeters in diameter.
Regional characteristics of the central cornea include the following:
- Decreased thickness
- More uniformity in the arrangement of the collagen fibers in the stroma
- Higher concentration of nerve endings
- Higher dependence on the pre-ocular tear film for nutrition
- Higher sensitivity to the effects of corneal edema secondary to corneal hypoxia
Regional characteristics of the peripheral cornea include the following:
- Increased thickness
- Loose ordering of the collagen fibers in the stroma
- Lower concentration of nerve endings
- Higher dependence on the capillaries at the limbus for nutrition
- Higher sensitivity to immune system responses
Corneal Endothelial Physiology
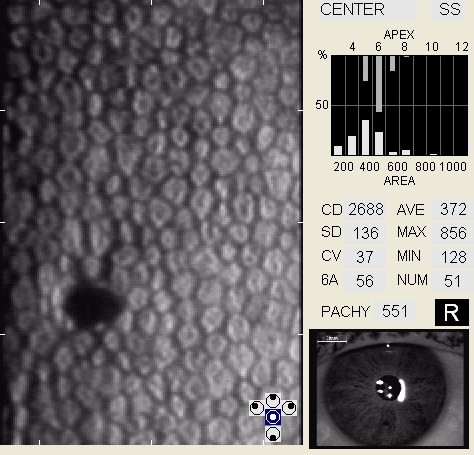
The corneal endothelium is a monolayer of 350,000 to 500,000 specialized cells that cover the posterior surface of the cornea. One of the endothelium’s physiological functions is to secrete a collagen matrix that forms Descemet’s membrane. At birth, Descemet’s membrane is approximately 3um thick. Throughout life, endothelial cells continue to synthesize and deposit additional collagen matrix. Over time, Descemets’s membrane grows thicker and thicker and by age 70 years, the average Descemet’s membrane is 13 um thick.
Corneal Hydration
Corneal hydration is one of the biomechanical properties of the anterior segment, and it is affected by several interdependent factors. To control corneal hydration, the endothelium forms an anatomical and physiological barrier to the aqueous that makes the structure semi-permeable. The ability to be semi-permeable is required because the endothelium must allow movement of nutrients into the cornea from the aqueous but maintain resistance to the effects of intraocular pressure.
| Age-Matched Normal Endothelial Cell Density | Endothelial Cell Density | |
|
|
The main goal of the diagnostic evaluation in a patient with endothelial corneal dystrophy is to accomplish the following:
- Determine the presence or absence of a corneal endotheliopathy
- Classify the corneal endotheliopathy as either primary or secondary
- Identify and exclude differential diagnoses
- Determine if the corneal endotheliopathy is clinically significant
- Prescribe a treatment program
Patient History
Patients with endothelial corneal dystrophy may present with any of the following clinical symptoms:
- None
- Reduced visual acuity
- Fluctuating visual acuity
- Glare
- Photophobia
- Halos around lights
- Foreign body sensations
- Contact lens intolerance
Corneal endotheliopathies are classified as either primary or secondary. In primary corneal endotheliopathies, the damage to the corneal endothelium is not associated with any other ocular or systemic disorder. In secondary corneal endotheliopathies, there is a recognizable ocular or systemic disorder which contributes to the damage of the corneal endothelium.
| Primary Corneal Endothelipathies | Seconday Corneal Endotheliopathies |
| Corneal guttata | Contact lens-induced endotheliopathy |
| Fuchs’ endothelial dystrophy | Iatrogenic endotheliopathy |
| Age-related endotheliopathy | Inflammation-induced endothliopathy |
Corneal Guttata — Classification
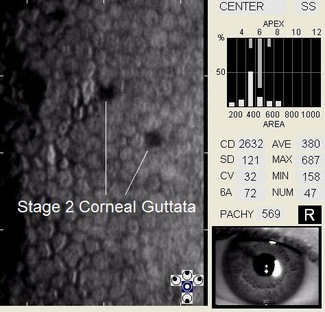
Corneal guttata is the most common primary corneal endotheliopathy and they are present in 70% of the population over 40 years old.
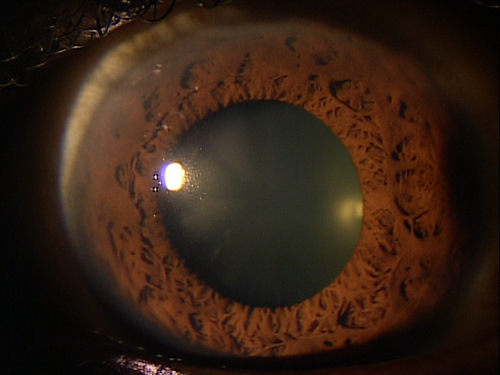
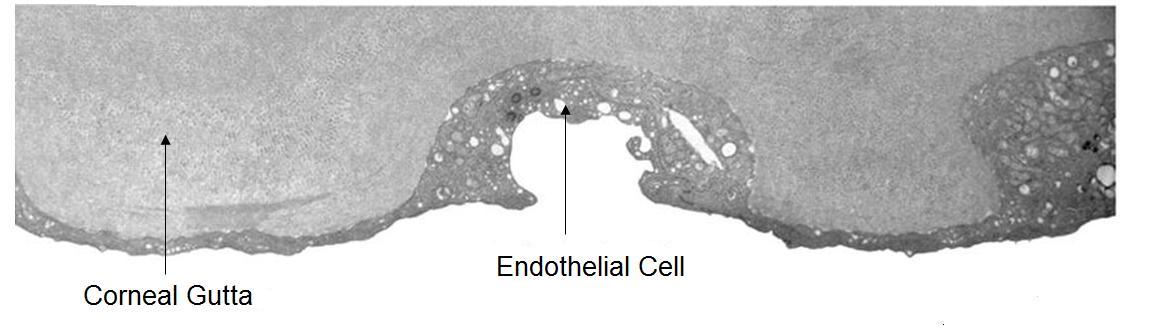
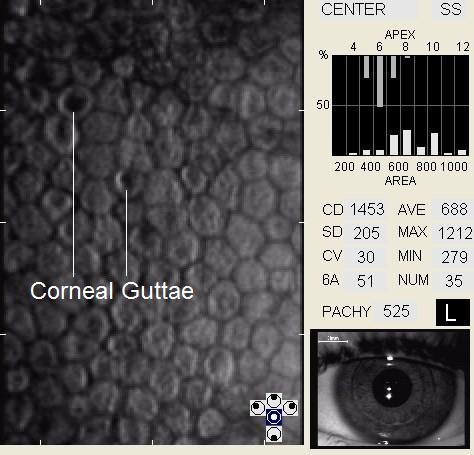
Corneal guttata are secretions of collagen from the endothelial cells that form a nodularity on the posterior surface of Descemet’s membrane. These nodules are created when endothelial cells under physiological stress secrete an altered basement membrane material that accumulates under the cells. The deposits of abnormal collagen eventually form a nodular-shaped lesion called a corneal gutta and the nodules are collectively referred to as corneal guttata.
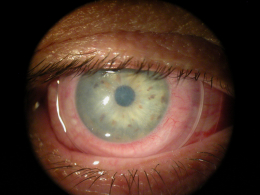
The clinical finding of corneal guttata is not specific; they may occur as part of the normal aging process, in corneal endothelial dystrophies, or secondary to ocular inflammation and trauma. Corneal guttata mainly affect the central region of the cornea and, when they are mild or moderate in their presentation, they usually have no effect on visual acuity.
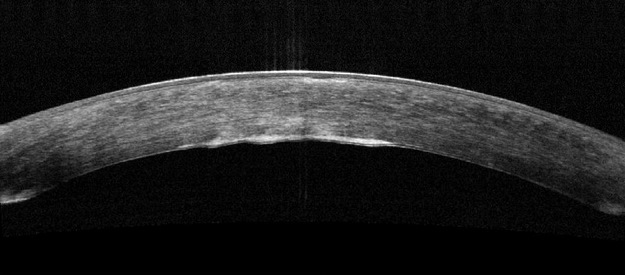
Posterior Cornea
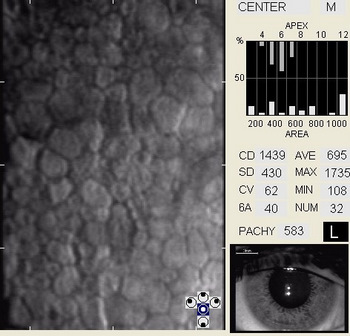
Corneal guttata formations usually do not change quickly. Studies of patients with Fuchs’ endothelial dystrophy found that few corneal guttata disappeared and few new guttata formations appeared within a 2-year period. Despite this slow rate of development and progression, corneal guttata may appear where none were previously present, may enlarge in number or size, or the may fuse together. When the corneal guttata are large and/or confluent, the endothelium may not function normally and visual acuity may be impaired.
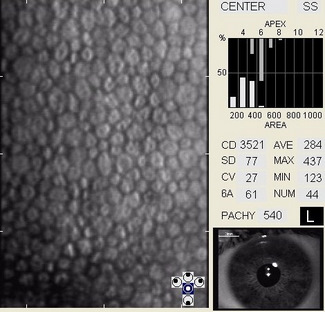
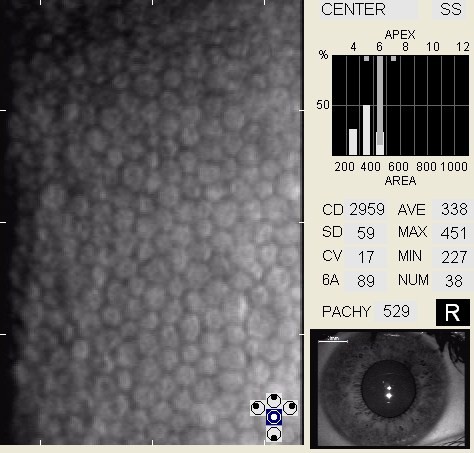
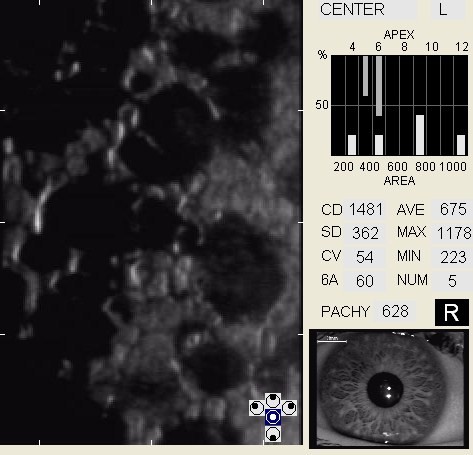
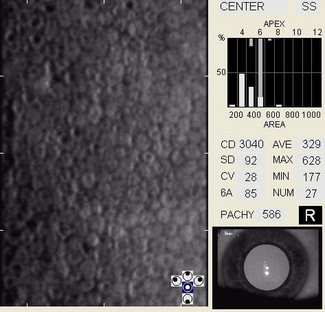
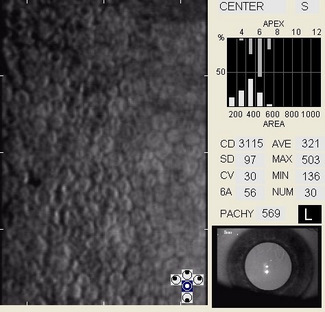
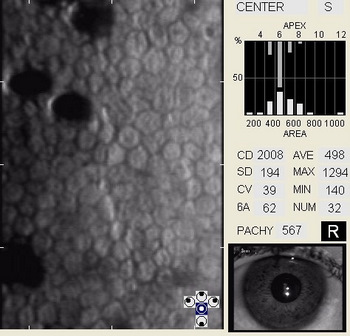
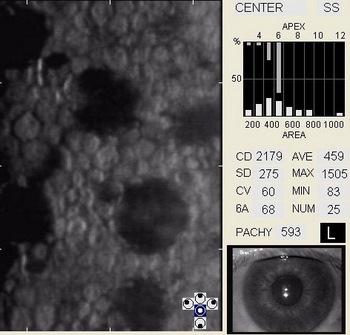
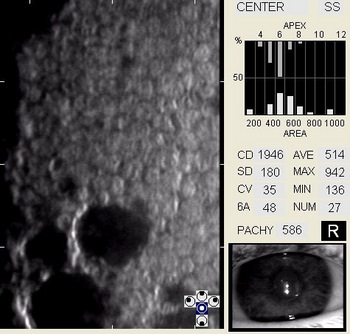
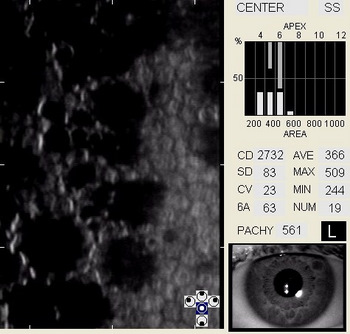
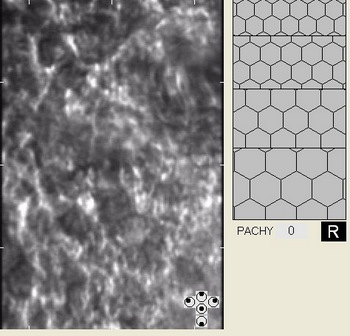
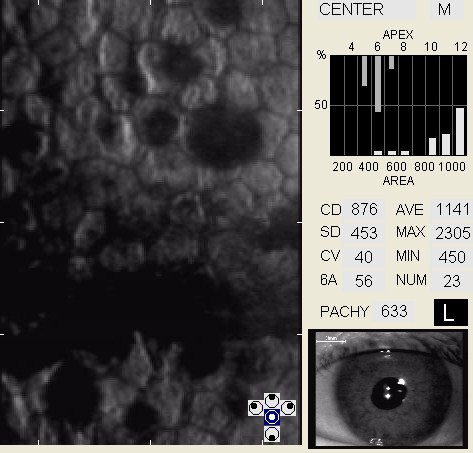
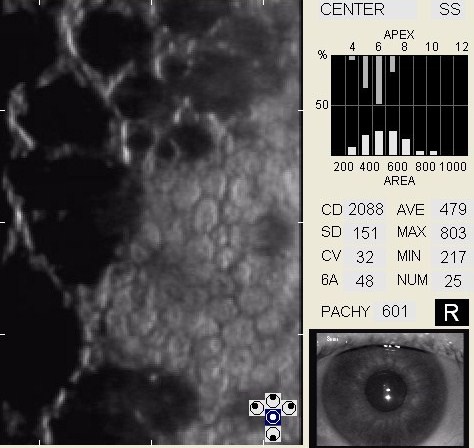
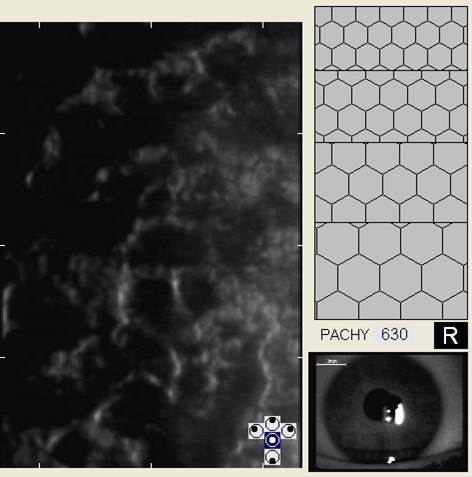
In patients with clinically significant endothelial disease, corneal guttata grow in size and number. The natural history of corneal guttata progression includes five specific stages of development, which can be discerned with specular microscopy.
 |
Corneal Guttata – Stage 1
|
Stage 1 corneal guttata in a 16-year-old female
Fuchs’ Endothelial Dystrophy — Classification
When confluent corneal guttata are present with clinically significant corneal edema, the condition is called Fuchs’ endothelial dystrophy. The disease is one of a group of opacifying disorders which develop in the absence of inflammation and it is characterized by a progressive loss of endothelial cell structure and function. Fuchs’ endothelial dystrophy affects 4% of the population over 40 years old.
Fuchs’ endothelial dystrophy is classified into three stages.
Stage 1 – The patient is usually asymptomatic. The identifying clinical characteristics include the following:
- The presence of corneal guttata at an early age
- An abnormal rate of polymegethism
- The presence of pleomorphism at an early age
- Endothelial pigment dusting
Stage 2 – The patient may have visual symptoms. The identifying clinical characteristics include the following:
- Corneal guttata formation increases
- Possible glare and decreased visual acuity, particularly upon awakening
- Stromal edema may develop secondary to the coalescence of corneal guttata
- Epithelial edema and folds in Descement’s membrane may develop after prolonged stromal edema
Stage 3 – The patient is usually symptomatic. The identifying clinical characteristics include the following:
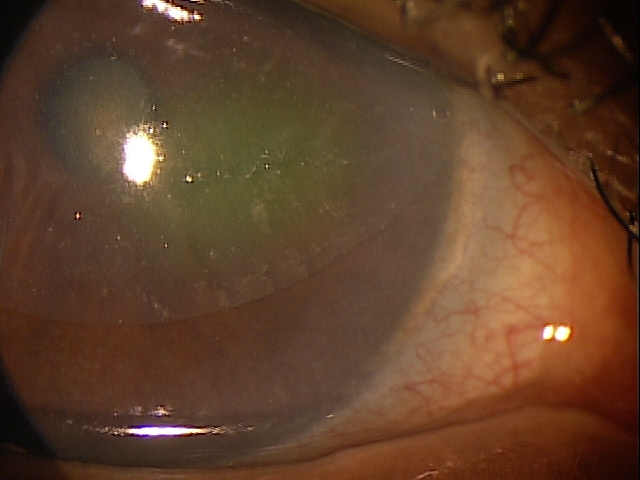
- The loss of endothelial function results in persistent stromal edema
- Advanced disease produces epithelial edema that is characterized by epithelial bullae, subepithelial bullae, and epithelial blisters (bullous keratopathy)
- Pain and photophobia occur secondary to corneal nerve damage
The treatment of patients with Fuchs’ endothelial dystrophy varies depending on the severity of the disease.
STAGE 1 DISEASE
Pharmocologic Treatment
Hyperosmotics
- The goal of treatment is to improve visual acuity by deturgescence of the stroma or epithelium. Compliance with topical hyperosmotic solutions can be challenging due to the burning sensations they often produce. Nighttime application of hyperosmotic ointment is an excellent if not preferred treatment.
Artificial Tears (FreshKote Ophthalmic Solution)
- The goal of treatment is to remove excess water from the epithelium by utilizing the high oncotic pressure of the solution
STAGE 2 DISEASE
Pharmocologic Treatment
Hyperosmotics
- The goal of treatment is to improve visual acuity by deturgescence of the stroma or epithelium. Compliance with topical hyperosmotic solutions can be challenging due to the burning sensations they often produce. Nighttime application of hyperosmotic ointment is an excellent if not preferred treatment.
Artificial Tears (FreshKote Ophthalmic Solution)
- The goal of treatment is to remove excess water from the epithelium by utilizing the high oncotic pressure of the solution
STAGE 3 DISEASE
Pharmocologic Treatment
Topical Ocular Hypotensives
In addition to hyperosmotics and FreshKote artificial tears, ocular hypotensive medications may be used to decrease intraocular pressure and corneal edema. These drugs reduce the fluid volume in the anterior chamber, thereby reducing stress on the endothelial pump mechanisms. These medications may be used even if the patient’s intraocular pressure is within a statistically normal range.
- Beta-blockers
- Adrenergic agonists
- Propagandists
- Docosanoids
- Cholinergic Agonists
Topical Non-Steroidal Anti-Inflammatory (NSAID)
Although these medications have no therapeutic effect on Fuchs’ dystrophy, they may be helpful in treating the pain associated with epithelial bullae and blisters. Because corneal melts have been associated with older NSAIDs, these medications should be avoided for long-term use.
- Ketorolac (e.g., Acuvail)
- Bromfenac (e.g., Prolensa)
- Nepafenac (e.g., Ilevro)
Topical steroids have not been shown to be of significant therapeutic benefit in Fuchs’ endothelial dystorphy and are not the best medication to relieve pain in this condition.
Mechanical Treatment
- Bandage contact lenses – (in patients with Fuchs’ endothelial dystrophy, overnight wear of soft contact lens can be used to decrease pain and improve visual acuity)
- Amniotic membrane insert
Surgical Treatment
Deep lamellar keratoplasty or one of its variations.
- DMEK — Descemet’s membrane endothelial keratoplasty
- DSEK — Descemet’s stripping endothelial keratoplasty
- DSAEK — Descemet’s stripping automated endothelial keratoplasty
DSEK is the most common surgical technique performed today. In this procedure, the surgeon peels away the posterior cornea (e.g., endothelium and Descemet’s membrane) and approximately 25% of the posterior stroma. A donor button of posterior stroma and posterior cornea are then implanted.
Amniotic Membrane Inserts:
1. Bourne WM. Biology of the corneal endothelium in health and disease. Eye 2003 Nov; 17(8):912-8.
2. Sullivan-Mee. The role of ocular biomechanics in glaucoma management. Rev Optom 2008 Oct 15;145(10): 49-54.
3. Edelhauser HF. The balance between corneal transparency and edema–the Proctor Lecture,. Invest Ophthalmol Vis Sci 2006 May; 47(5):1754-67. (All referenced photographs have been reprinted with the permission of the author.)
4. Taravella M, Walker M. Emedicine Postoperative corneal edema. Available at: www.emedicine.com/oph/topic64.htm (Accessed March 16, 2014).
5. Phillips C, Laing R, Yee R. Specular Microscopy. In: Krachmer JH, Mannis MJ, Holland EJ (eds). Cornea, 2nd ed. Philadelphia: Elsevier Mosby, 2005:261-77.
6. Liesegang TJ. Physiologic changes of the cornea with contact lens wear. CLAO J 2002 Jan;28(1): 12-27
7. McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008 Jan;27(1):1-16
8. Corneal endothelial photography. Three-year revision. American Academy of Ophthalmology. Ophthalmology 1997 Aug;104(8):1360-5.
9. Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol 2007 Sep;91(9):1165-9.
10. Kitagawa K, Fujisawa A, Mizuno T, Sasaki K. Twenty-three cases of primary cornea guttata Jpn J Ophthalmol 2001 Jan-Feb;45(1):93-8.
11. Giasson CJ, Solomon LD, Polse KA. Morphometry of corneal endothelium in patients with corneal guttata. Ophthalmology 2007 Aug;114(8):1469-75.
12. Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea 1988;7(1):2-18.
13. Singh D, Singh R. Emedicine: Dystrophy, Fuchs Endothelial. Available at: www.emedicine.com/oph/topic91.htm (Accessed March 16, 2014).
14. Zhang C, Bell WR, Sundin OH, et al. Immunohistochemistryand electron microscopy of early-onset Fuchs’ corneal dystrophy in three cases with the same L450W COL8A2 mutation. Trans Am Ophthalmol Soc 2006;104:85-97.
15. Mishima S. Clinical investigations on the corneal endothelium-XXXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol 1982 Jan;93(1):1-29.
16. Sibug ME, Datiles MB, Kashima K, et al. Specular microscopy studies on the corneal endothelium after cessation of contact lens wear. Cornea 1991 Sep;10(5):395-401.
17. Laing RA, Oak SS, Leibowitz HM. Specialized Microscopy of the Cornea: Specular Microscopy. In: Leibowitz HM, Waring GO (eds). Corneal Disorders: Clinical Diagnosis and Management, 2nd ed. Philadelphia: W.B. Saunders Company, 1998:83-122.
18. Samudre SS, Lattanzio FA, Willimas PB, Sheppard JD. Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther 2004 Dec;20(6):533-47.
19. Miller WL. Caring for bullous keratopathy. Contact Lens Spect Sept 200621(9):52.
371.57
Endothelial corneal dystrophy
76513
Anterior segment ultrasound
76514
Corneal pachymetry
92015
Refraction
92285
External ocular photography
92286
Specular endothelial microscopy
92071
Bandage contact lens
65778
Amniotic membrane placement
Occurrence
More common and with greater severity in women by a ratio of 3:1.




 Print | Share
Print | Share