Case Report ID: 23
Title
Elevated-Tension Open-Angle Glaucoma with Isolated-Check VEP and pERG Abnormalities
Category
Glaucoma (8)
Description
Glaucoma is an optic neuropathy showing distinctive changes in optic nerve morphology without associated pallor.
Glaucoma is an optic neuropaty showing distinctive changes in optic nerve morphology without associated pallor. The term “glaucoma” refers to a group of chronic, progressive optic neuropathies that have in common characteristic morphologic changes at the optic nerve and retinal nerve fiber layer.
Case Report
- A 42-year-old black man presented with a chief complaint of decreased vision
- Case history described a subjective decrease in vision over the past year
- The patient had been
Conclusion
Although it may be very easy to conclude that this patient has glaucoma, the decisions regarding the problems associated with glaucoma and especially advanced glaucoma are very important and often difficult to make.
History of Present Illness
- Associated Signs and Symptoms: none
- Location: n/a
- Duration: n/a
- Quality: n/a
- Context: n/a
- Severity: n/a
- Timing: n/a
- Modifiers: n/a
Review of Systems
- The patient reported that he was in good health and taking no medications
Past, Family and Social History
- Non-contributory
Uncorrected Distance Visual Acuity
- 20/15 in the right eye
- 20/15 in the left eye
Normal Examination Findings
- Mental status
- General medical observation
- Gross visual fields
- Pupils
- Basic sensorimotor examination
- External examination
- Adnexal examination
- External ocular examination with biomicroscopy
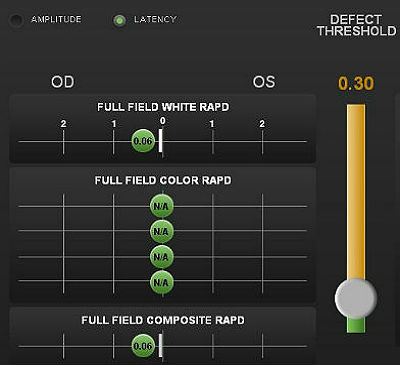
Pupillary Examination
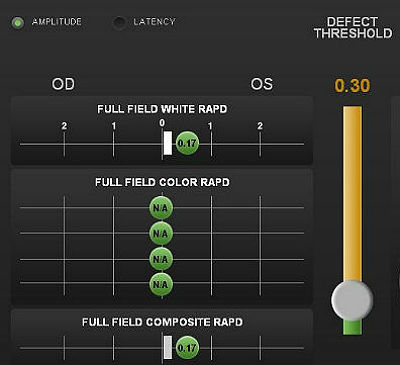
Pupillary reactivity testing using a RAPDx automated pupillographer was abnormal.
- A relative afferent pupillary defect was detected in the left eye
- Index-of-defect number was 0.17 for the amplitude of the pupillary response in the left eye
- Number is calculated by determining the log difference in amplitude between the pupillary responses
- Numbers above 0.30 are considered abnormal
- The higher the number, the more likely pathology is present in the neural light reflex pathway
 |
Intraocular Pressure Measurements
- 28 mm Hg in the right eye
- 24 mm Hg in the left eye
- Population studies indicate that the average IOP is 15.5 mm Hg +/- 2.6 mm Hg.
Some studies suggest that there is a relationship between between the amount of intraocular pressure asymmetry and the likelihood of having glaucoma.
- A difference in IOP of 0 mm Hg correlates with a 0.5% chance of having glaucoma
- A difference in IOP of 3 mm Hg correlates with a 10% chance of having glaucoma
- A difference in IOP of 6 mm Hg correlates with a 50% chance of having glaucoma
- A difference in IOP of more than 6 mm Hg correlates almost 100% of the time with patients having glaucoma
As an aside, this patient was seen two years earlier with IOP readings 24 mm Hg in the right eye and 22 mm Hg in the left eye.
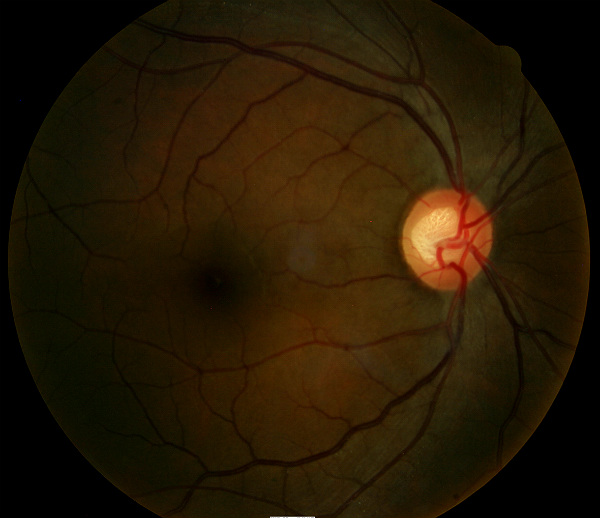
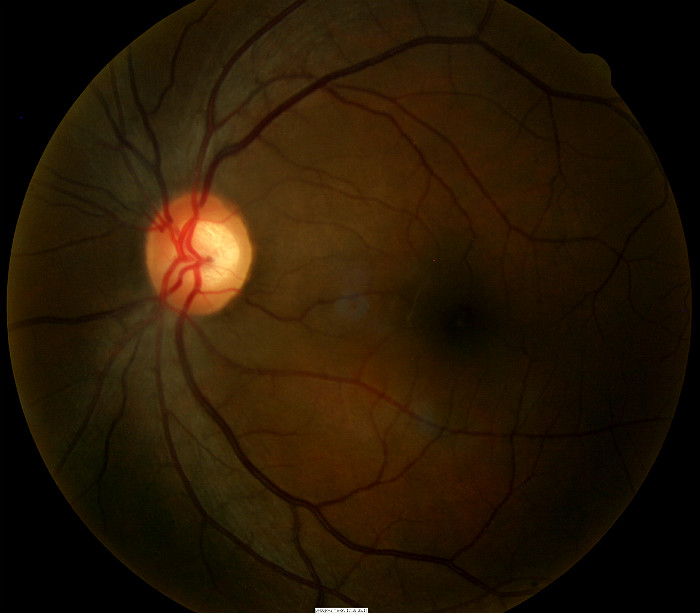
Ophthalmoscopy
Right Eye
- Cup-to-disc ratio = .70/.70
- Vertical elongation of optic cup
- Moderate cup depth
Left Eye
- Cup-to-disc ratio = .70/.65
- Vertical elongation of optic cup
- Moderate cup depth
Clinical Diagnosis
The clinical diagnosis is a determination based on the knowledge obtained from the patient’s medical history and from the results of the eye examination alone, without the benefit of diagnostic tests or procedures.
The patient’s clinical diagnosis is elevated-tension open-angle glaucoma based on the following clinical findings:
- Elevated intraocular pressure measurements
- Anomalous optic nerve appearance
Treatment Plan
To gather the clinical information required to treat elevated-tension open-angle glaucoma, a diagnostic and treatment program is initiated.
- Determination of different types of diagnoses
- Selection of one or more treatment options
Glaucoma is a disease that must be classified by type to be treated properly. After a clinical diagnosis has been determined, the diagnostic process continues with a process that involves the identification and exclusion of differential diagnoses. The differential diagnosis process allows the eye doctor to distinguish between two or more diseases with similar signs and symptoms by systematically comparing their signs and symptoms.
Differential Diagnoses
The first differential diagnosis that needs to be determined in this patient is glaucoma vs. some other retinal or neurological disease. The list of possible diseases would include any pathology that produces an asymmetric loss of neuroretinal rim tissue, a corresponding retinal nerve fiber layer defect and an associated visual field defect.
The following diseases share some of the clinical signs and symptoms of glaucoma.
- Retinal detachment
-
Pituitary tumors and other neurological conditions
-
Ischemic optic neuropathy
-
Vascular disease
-
Drusen of the optic disc
-
Congenital disc anomalies
Retinal detachments and drusen of the optic disc would be easily identified during routine ophthalmoscopy. Pituitary tumors producing morphologic changes in the optic nerve should be associated with characteristic bitemporal visual field defects. The patient did not have a history of vascular disease and no retinal vascular findings that would typically be associated with more advanced systemic vascular disease.
Ordering Diagnostic Tests
When additional clinical information is needed to complete the differential diagnostic process, diagnostic tests and procedures are ordered. The performance of these tests and procedures leads to the completion of the differential diagnostic process while simultaneously initiating the treatment program.
Based on the clinical diagnosis of glaucoma, the following diagnostic tests and procedures were ordered at the conclusion of the eye examination:
- Refraction
- Retinal nerve fiber layer scan
- Visual field examination
- Gonioscopy
- Fundus photography
- Color vision examination
- Visual evoked potential testing
The decision to order and perform additional testing is totally based on the concept of medical necessity which can only be determined by the examining optometrist or ophthalmologist.
INITIAL VISIT
Refraction
Measuring visual acuity is a method of evaluating functional vision loss.
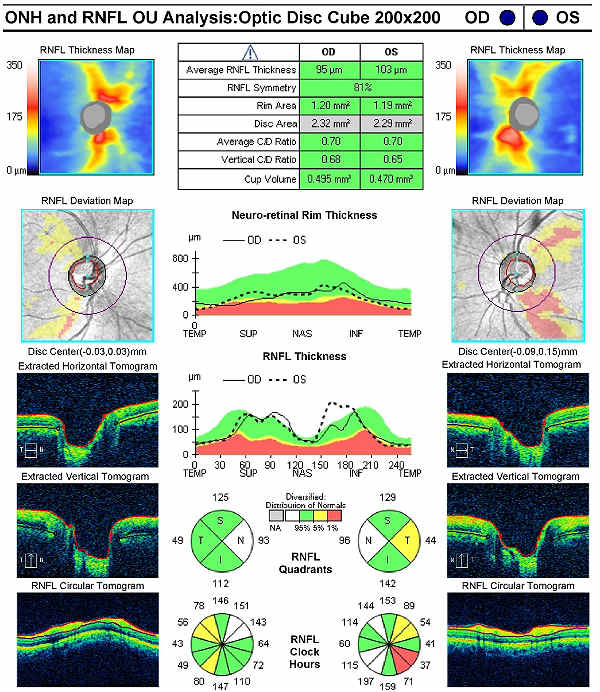
Retinal Laser Scan – Nerve Fiber Layer
- Glaucoma-induced retinal nerve fiber layer thinning may precede vision field loss
- Glaucoma-induced retinal nerve fiber layer thinning has a predilection for the inferior and superior bundles
- Measuring the thickness of the retinal nerve fiber layer can be accomplished by using the CIRRUS OCT manufactured by Carl Zeiss Meditec as well as other scanning laser instruments
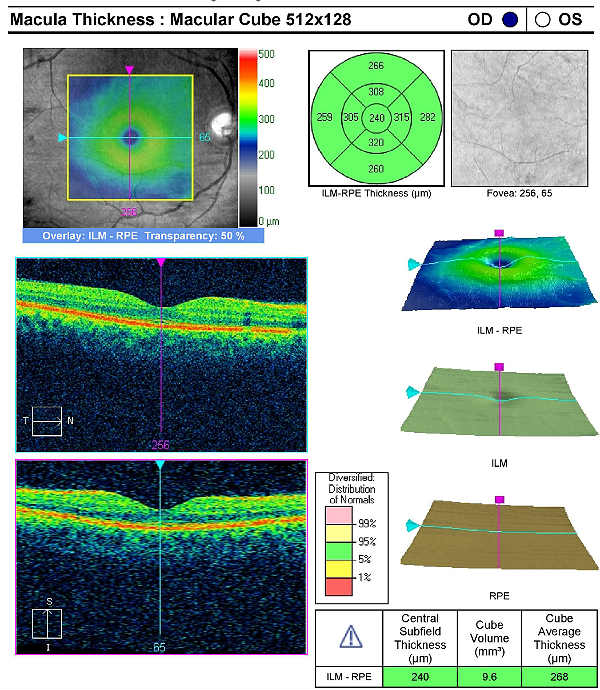
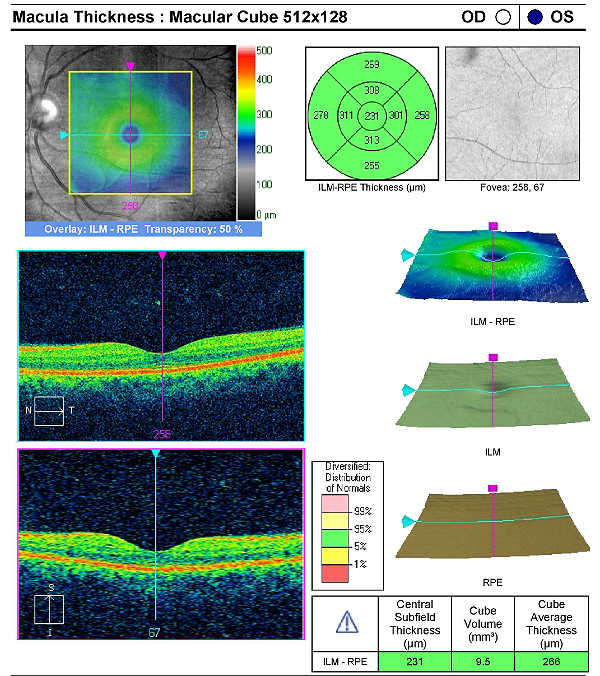
Retinal Laser Scan — Macula
Measuring retinal thickness and detailed evaluation of retinal anatomy is a means to evaluate overal retinal health and the procedure can be accomplished with an OCT manufactured by Carl Zeiss Meditec.
 |
Right Eye
|
|
Left Eye
|
 |
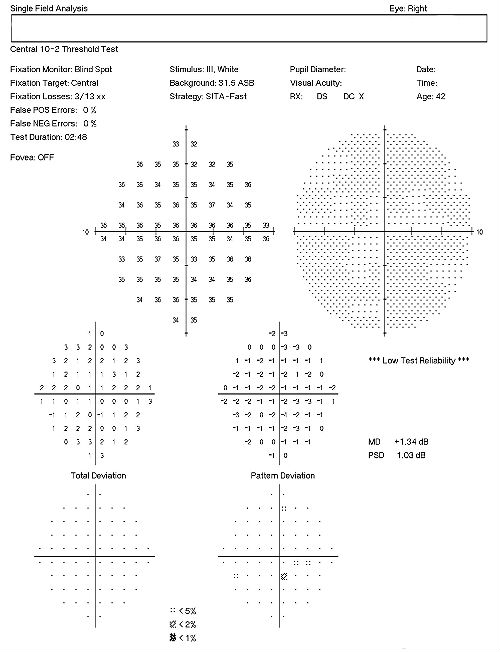
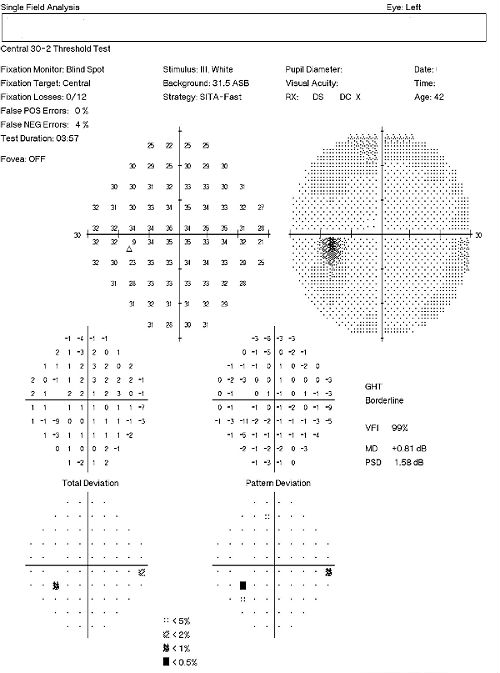
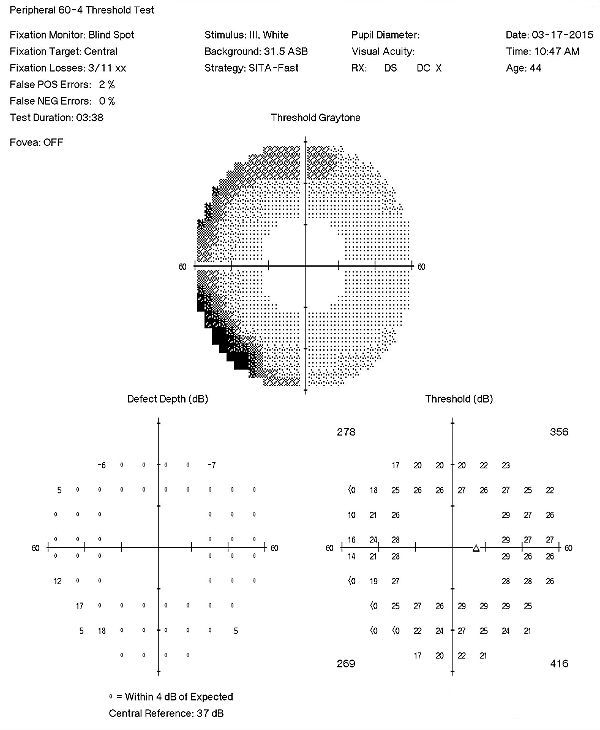
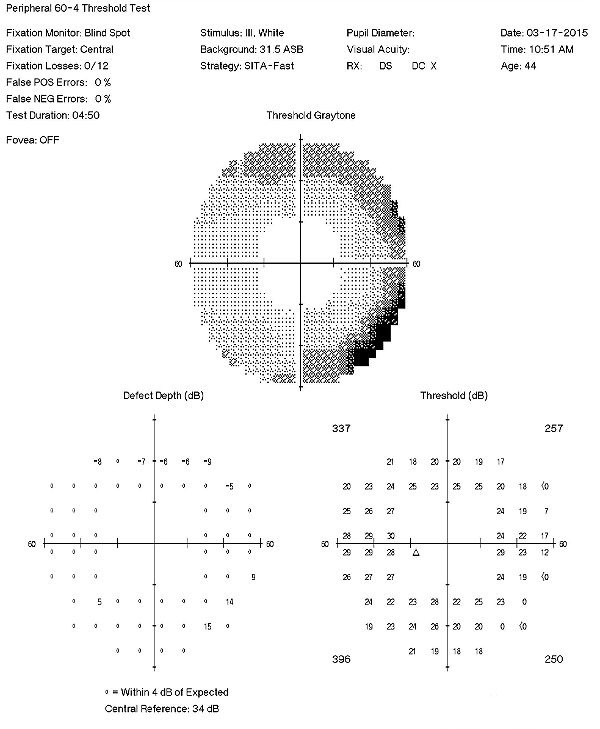
Visual Field Examination
- Automated threshold perimeters measure the visual field by plotting the threshold luminance value of the patient in various locations in the visual field
- The luminance of the light stimulus is represented by non-specific units of measurement called decibels (dB)
- Glaucoma produces several changes in the visual field, and one of these changes occurs as a widespread, non-descript loss of retinal sensitivity
- In Tranquair’s “Hill of Vision” concept, loss of retinal sensitivity represents a reduction in the height of the hill
- The diffuse loss of retinal sensitivity should be considered highly diagnostic of glaucoma when it is asymmetric and correlates with asymmetric changes in intraocular pressure or disc appearance
- In most cases, the loss of sensitivity occurs in characteristic patterns and locations (e.g., nasal step, arcuate scotoma, paracentral scotoma) that often correlate with changes in the optic nerve and/or retinal nerve fiber layer.
Visual Field Examination (Subsequent Visit)
A 60-4 testing strategy sometimes gives a different perspective compared to traditional 30-2 field examinations
- xxx
- xxx
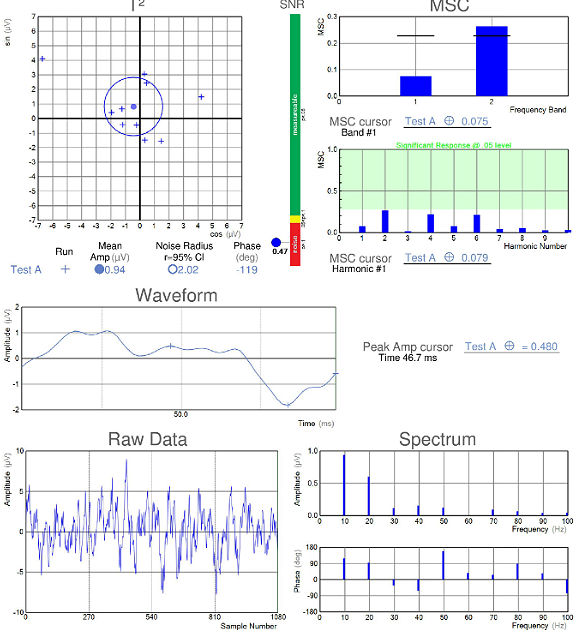
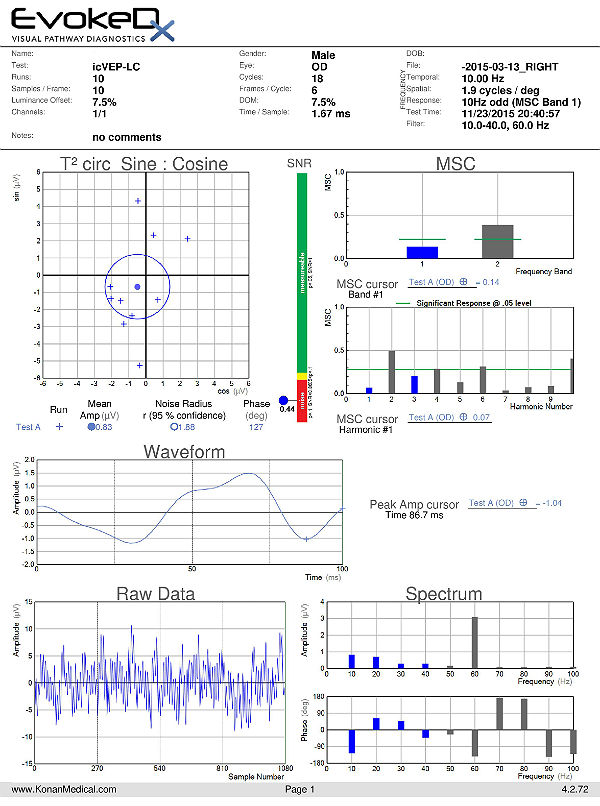
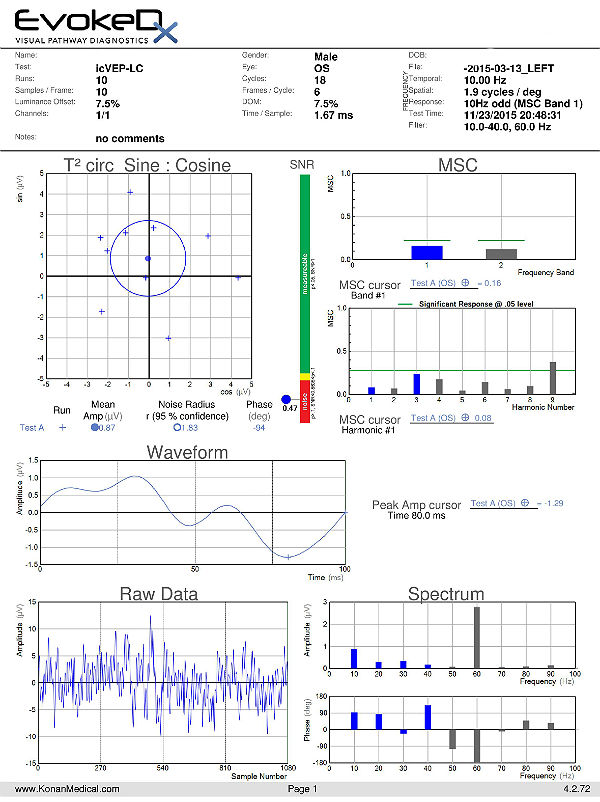
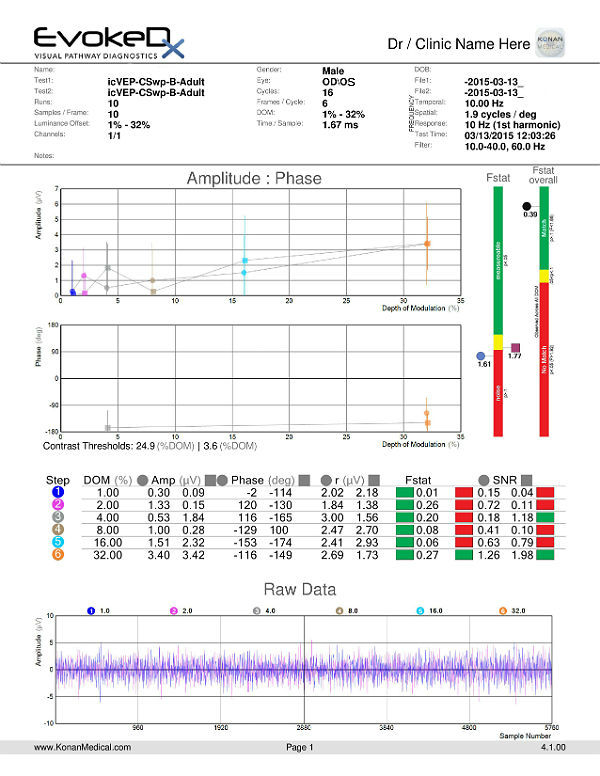
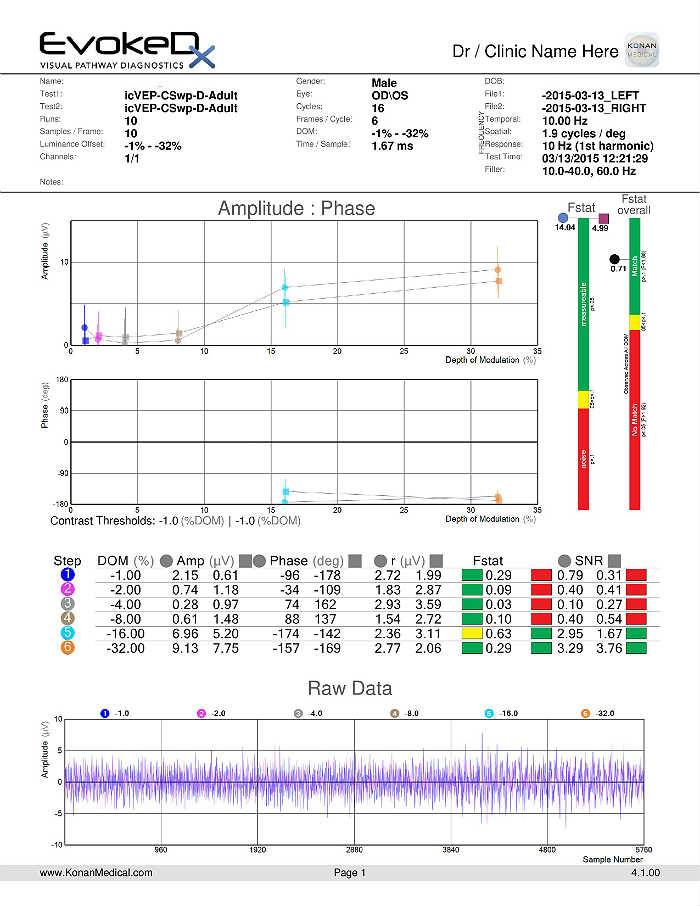
Visual Evoked Potential Testing
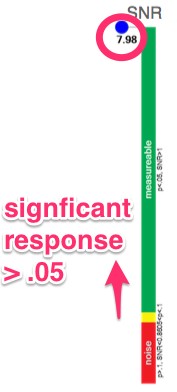
- Visual evoked potential testing evaluates the integrity of the afferent visual sensory system
- Technologies have been designed to tease out RGC functional losses, and in particular, to the Magnocelluar pathway which conveys primarily low luminance contrast information (eg. FDT subjective perimetry). Detection of loss of low contrast function is though to be of value with early glaucomatous damage.
- The EvokeDx (Konan Medical) system features a library of test strategies including a patented, low-contrast, high temporal frequency, isolated bright or dark-check pattern that is sinusoidally modulated, on and off, against a uniform grey (50cd/m2) background, producing an appearance – disappearance phenomena to the patient. This high temporal frequency pattern, “icVEP”, is designed to assess the low-contrast processing in the central vision system thought to be affected early on in the development of glaucoma and other conditions.
- The Magnocellular ON pathway contains the largest cells (M-cells) in the human visual system, and therefore, they are likely to be the most metabolically active and susceptible to disease. Bright checks are designed to target the Magnocellular ON and dark checks, the OFF pathways.
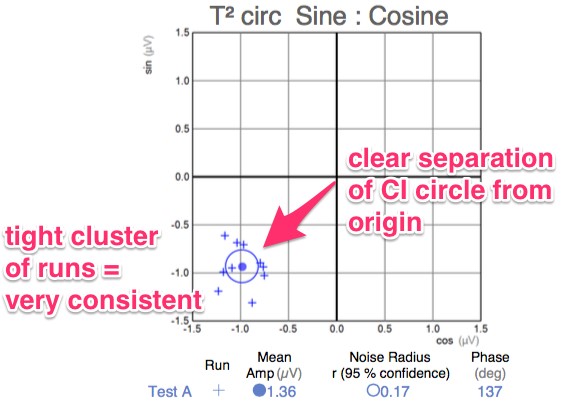
- A series of very short “runs” of about 2 seconds (icVEP-LC) or 12 seconds sweeps (icVEP-CSwp dark or bright), create the time-domain data packages that are averaged and then Fourier-transformed to the frequency domain
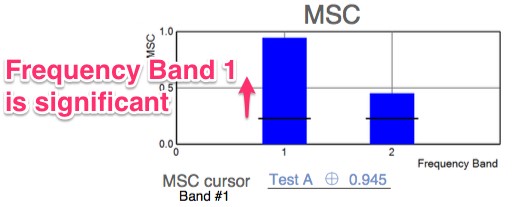
- Multi-variant, Fourier-transformed, analytic tools are then used to assess frequency domain signal and noise information derived from the entire waveform rather than an assessment based upon just one or two time domain latency values
icVEP-CSwp-B (Bright Checks)
icVEP-CSwp-D (Dark Checks)
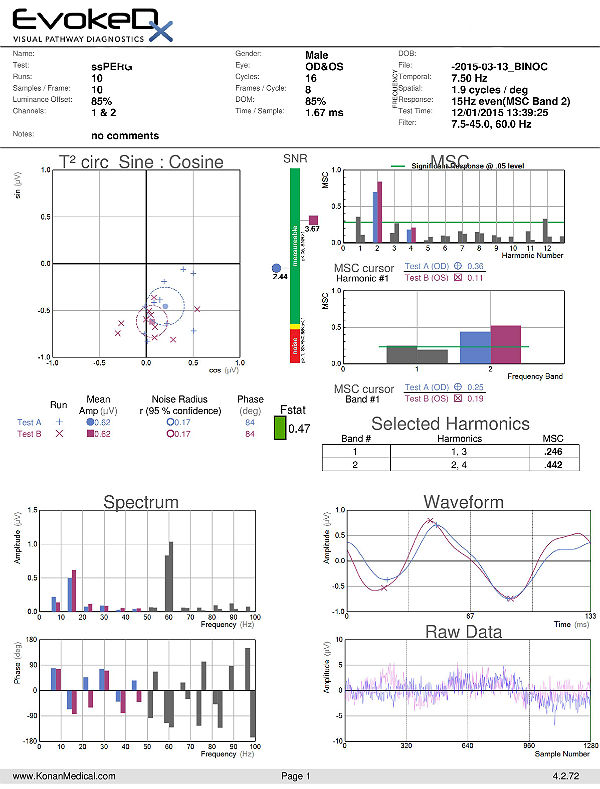
Electroretinography
- Electroretinogram testing evaluates the integrity of the retina
- The procedure can be accomplished using the EvokeDx device manufactured by Konan Medical
- Specialized pattern electroretinogram (PERG) test uses a contrast-reversing pattern for the stimulus to produce information about ganglion cell function
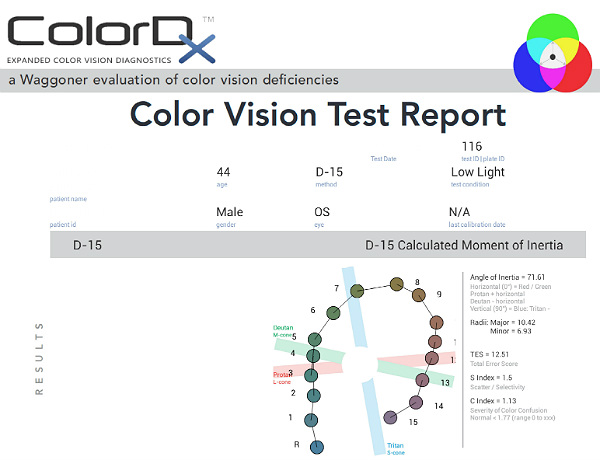
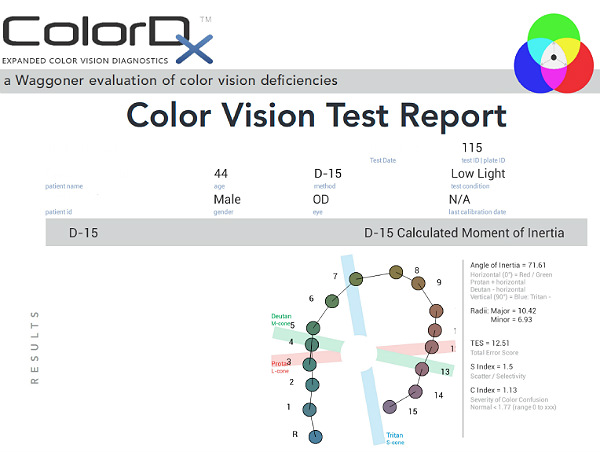
Color Vision Examination
An extended color vision examination measures the combined function of the retina, the optic nerve and the intracranial visual pathway and is used clinically to detect or monitor color vision loss due to disease at any of these locations. The procedure can be accomplished using the ColorDx software from Konan Medical.
 |
Konan’s ColorDx Color Vision Testing System
|
Some patients with glaucoma develop an acquired dyschromatopsia during the natural history of the disease.
- A generalized loss of chromatic discrimination affects 20% – 30% of patients with glaucoma
- Color vision defects may precede visual field loss in patients with glaucoma
- Some patients only develop color vision loss in advanced disease
All of the diagnostic test results confirmed the initial diagnosis of elevated-tension open-angle glaucoma. Additional diagnostic tests for glaucoma such as serial tonometry, provocative glaucoma testing, or extended ophthalmoscopy were not medically necessary on this patient’s initial visit. Photodocumentation of the optic nerve should be performed at some follow-up visit.
According to Current Procedural Terminology, when eye doctors perform ophthalmological examinations, the complexity of medical decision-making is not separated from the examining techniques used. As a guideline to assist eye doctors in enhancing their medical decision-making skills, consider that the complexity of medical decision-making involves three components.
The first component concerns the number of possible diagnoses and treatment options that must be considered. Modern glaucoma diagnosis involves the determination of structural damage to the eye and/or functional loss to the visual system. Once these assessments are made, the glaucoma must be classified by type.
The second component concerns the amount and complexity of medical records and diagnostic tests that have to be obtained, reviewed and analyzed. In addition to an eye examination, this visit required the review and analysis of a subjective refraction, a retinal laser scan, a threshold visual field examination, and a gonioscopic examination of the anterior chamber.
Third, the complexity of medical decision-making is affected by the risk of significant complications and/or morbidity associated with advanced glaucoma and the risks involved in any treatment options. This patient’s glaucoma was classified as a severe problem where the risk of total vision loss without treatment was significant. In addition, because the treatment plan would involve chronic pharmaceutical treatment with the potential for ocular and systemic side effects, the pharmacology complications had to be considered when deciding on the initial treatment options.
Treatment Guidelines
To begin the treatment of glaucoma, intraocular pressure must be lowered and an initial target pressure range must be established. A general guideline is to base the initial pressure range on the category of damage to the visual system.
- Mild damage needs a 20-30% reduction in pre-treatment intraocular pressure as the initial target pressure range
- Moderate damage needs a 30-40% reduction in pre-treatment intraocular pressure as the initial target pressure range
- Advanced damage needs a 40-50% reduction in pre-treatment intraocular pressure as the initial target pressure range
Doctors should always remember that the goal in glaucoma therapy is preventing future or additional vision loss, not chasing some arbitrary pressure range.
Treatment Program
Modern glaucoma therapy generally utilizes prostaglandins as the primary therapy. These medications typically have the greatest efficacy. The first goal of treating glaucoma is to achieve the lowest possible intraocular pressure on a single agent. Monotherapy with prostaglandins improves patient compliance, may decreases costs, and improves the safety profile and tolerability of using topical medications. These drugs reduce intraocular pressure by increasing uveoscleral outflow. Intraocular pressure reduction averages almost 30%, sometimes more and sometimes minimal to no effect.
Clinically significant conjunctival hyperemia is the most common side effect of prostaglandin therapy. Another possible ocular side effect of prostaglandins includes changes in iris color after several months of use. In these patients, the drug produces an increase in the amount of iris stromal pigmentation. Over time, eyelashes can lengthen, thicken, and darken – a side effect known as hypertrichosis.
The only serious ocular side effect is a 3% increased risk of developing cystoid macular edema. A history of uveitis is a relative contraindication, as prostaglandins produce an increased risk of reactivating the inflammation. The systemic side effects of topical prostaglandins are minimal.
Avoiding medicolegal liability if complications arise is an important part of a modern ophthalmic practice. This avoidance is best accomplished by making sure that you have obtained the patient’s informed consent to treat their glaucoma. Once you have made the patient fully aware that using topical glaucoma medicine involves the risks of side effects, you can proceed with the treatment plan.
After obtaining the patient’s informed consent to treat her glaucoma, Travatan Z ophthalmic solution was prescribed 1 drop at bedtime in both eyes. A target pressure was set at OD 21 mm Hg and OS as low as possible. It would be typically unrealistic to expect any monotherapy to achieve the desired target endpoints, although this can rarely occur with prostaglandin therapy.
Next examination scheduled for 2 weeks. Full pressure reduction with prostaglandins is typically not achieved until 30 days but Travatan Z has been shown to reach near full potential in as little as two weeks.
Discussion
This patient’s clinical history supports the modern understanding of the pathophysiology of glaucoma. We know that glaucoma is an optic neuropathy. Structural damage to the retinal nerve fiber layer is usually the first milestone in the natural history of glaucoma. The loss of retinal nerve fibers is closely followed by changes in the appearance of the optic disc. Functional loss, which is documented by abnormal visual field testing, abnormal visual evoked potential testing or loss of visual acuity, usually occurs later in the natural history of the disease. In this case, there is advanced functional damage to the left eye.
On initial examination, the damage to the optic disc was advanced in the left eye, and its prognosis for continued good vision ten or twenty years from now is poor. Without significant therapeutic effect from medication, it is likely this left eye will require laser trabeculoplasty (SLT). The advanced damage and fragile nature of the remaining nerve fibers in the left optic nerve is likely to eliminate consideration of the risks associated with a filtering procedure. The right eye, while having less damage to the retinal nerve fibers and only mild visual field defects, had a very abnormal retinal laser scan and elevated IOPs. The abnormal retinal laser scan suggests that this patient’s right eye is in the interval between the measurement of structural damage from glaucoma and the measurement of functional vision loss from glaucoma. This gap, up to five years in some patients, often delays and confuses eye doctors in trying to diagnose glaucoma or to assess its severity.
Examples of Normal Response
Based on the patient history, the nature of the presenting problem, and my own clinical judgement this patient needed an evaluation of the complete visual system.
- Perform the eye examination that is medically necessary
- Provide the diagnostic tests or services that are medically necessary
- Properly document the services provided
- Code from the documentation
- Report the services to the payor
Multiple Procedure Payment Reduction
Effective January 1, 2013, there is a small reduction in payment from Medicare if certain multiple procedures are billed on the same day. The fee for the technical component of the diagnostic test for the second and subsequent tests will be reduced by 20%. The second diagnostic test and subsequent tests should be reported with a -51 modifier. Professional services such as gonioscopy, extended ophthalmoscopy, serial tonometry and provocative glaucoma testing are excluded from this policy. Visual evoked potential testing is excluded from this policy.
Modifier 51
This modifier is used to identify the secondary procedure or when multiple procedures are performed on the same day by the same provider. List the major primary procedure first and append the modifier to the subsequent procedures. The primary procedure is the one with the highest dollar value.
INITIAL VISIT
| Diagnosis Code | Procedure Code | Modifier | Quantity | Payor | Amount Allowed |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92004 - Medical eye examination | 1 | Aetna | 74.13 | |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92083 - Visual field examination | 51 | 1 | Aetna | 59.63 |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92250 - Fundus photography | 1 | Aetna | 53.45 | |
| H47.233 - Glaucomatous optic atrophy | 95930 - Visual evoked potential | 1 | Aetna | 135.58 | |
| Total | $322.79 |
SUBSEQUENT VISIT
| Diagnosis Code | Procedure Code | Modifier | Quantity | Payor | Amount Allowed |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92012 - Medical eye examination | 1 | Aetna | 63.24 | |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92083 - Visual field examination | 51 | 1 | Aetna | 59.63 |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92275 - Electroretinography | 1 | Aetna | 167.58 | |
| H47.233 - Glaucomatous optic atrophy | 95930 - Visual evoked potential | 1 | Aetna | 135.58 | |
| H40.1131 - Open-angle glaucoma, bilateral, mild stage | 92283 - Color vision examination | Aetna | 34.56 | ||
| Total | $460.59 | ||||
| Total for both visits | $783.38 |
H40.1131
Primary open-angle glaucoma,
bilateral, mild stage
H47.233
Glaucomatous optic atrophy,
bilateral
365.11
Primary open-angle glaucoma
92133
Retinal nerve fiber laser scan
92083
Visual field examination
92283
Color vision examination
92250
Fundus photography
92225
Extended ophthalmoscopy
92226
Subsequent ophthalmoscopy
92020
Gonioscopy
95930
Visual evoked potential
92275
Electroretinography
92100
Serial tonometry
92132
Anterior segment imaging
76513
Anterior segment ultrasound
76514
Corneal pachymetry




 Print | Share
Print | Share