Case Report ID: 19
Title
Elevated-Tension Open-Angle Glaucoma; Moderate Damage
Category
Glaucoma (8)
Description
This case describes the diagnosis and treatment of a patient with elevated-tension open-angle glaucoma.
Glaucoma is an optic neuropathy showing distinctive changes in optic nerve morphology without associated pallor. The term “glaucoma” refers to a group of chronic, progressive optic neuropathies that have in common characteristic morphologic changes at the optic nerve and retinal nerve fiber layer.
The glaucomas are associated with the following clinical features:
- Aqueous outflow resrictions
- Unphysiologic intraocular pressure
- Abnormal ocular perfusion
- Abnormal rate of apoptosis
- Progressive retinal ganglion cell loss
- Characteristic changes in optic nerve anatomy
Case Report
- A 40-year-old black man returned to the office for a “routine” eye examination
- Case history revealed that the only visual complaint was mild difficulty seeing at night
- The patient wore prescription eyeglasses and wanted to see if a new prescription would improve his vision
Conclusion
Glaucoma is a diagnosis of exclusion. Most patients will not present with visual complaints or symptoms the are suspicious for glaucoma.
History of Present Illness
- Associated Signs and Symptoms: none
- Location: n/a
- Duration: n/a
- Quality: n/a
- Context: decreased vision is more noticeable when driving at night
- Severity: mild decrease in vision
- Timing: vision seems to be getting worse over time
- Modifiers: none
Review of Systems
- The patient reported that he was in good health and taking no medications
Past, Family and Social History
- Non-contributory
Uncorrected Distance Visual Acuity
- 20/30 in the right eye
- 20/30 in the left eye
Best Corrected Distance Visual Acuity
- 20/20 in the right eye
- 20/20 in the left eye
Normal Examination Findings
- Mental status
- General medical observation
- Gross visual fields
- Pupils
- Basic sensorimotor examination
- External examination
- Adnexal examination
- External ocular examination with biomicroscopy
Intraocular Pressure Measurements
- 20 mm Hg in the right eye
- 27 mm Hg in the left eye
Population studies indicate that the average IOP is 15.5 mm Hg +/- 2.6 mm Hg.
Some studies suggest that there is a relationship between between the amount of intraocular pressure asymmetry and the likelihood of having glaucoma.
- A difference in IOP of 0 mm Hg correlates with a 0.5% chance of having glaucoma
- A difference in IOP of 3 mm Hg correlates with a 10% chance of having glaucoma
- A difference in IOP of 6 mm Hg correlates with a 50% chance of having glaucoma
- A difference in IOP of more than 6 mm Hg correlates almost 100% of the time with patients having glaucoma
Ophthalmoscopy
- Cup-to-disc ratio = .60/.55 in the right eye
- Cup-to-disc ratio = .70/.65 in the left eye
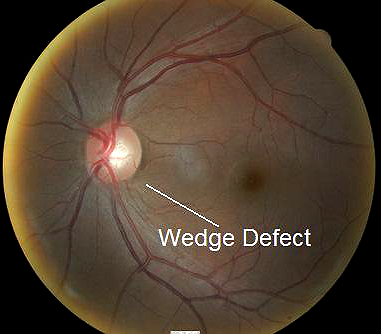
- Wedge-shaped nerve fiber layer defect with its apex in the inferior region of the optic disc
Clinical Diagnosis
The clinical diagnosis is a determination based on the knowledge obtained from the patient’s medical history and from the results of the eye examination alone, without the benefit of diagnostic tests or procedures.
The patient’s clinical diagnosis is elevated-tension open-angle glaucoma based on the following clinical findings:
- Elevated intraocular pressure measurements
- Asymmetric intraocular pressure measurements
- Optic neuropathy in the left eye
Treatment Plan
To gather the clinical information required to treat elevated-tension open-angle glaucoma, a diagnostic and treatment program is initiated.
- Determination of different types of diagnoses
- Selection of one or more treatment options
Glaucoma is a disease that must be classified by type to be treated properly. After a clinical diagnosis has been determined, the diagnostic process continues with a process that involves the identification and exclusion of differential diagnoses. The differential diagnosis process allows the eye doctor to distinguish between two or more diseases with similar signs and symptoms by systematically comparing their signs and symptoms.
Differential Diagnoses
The first differential diagnosis that needs to be determined in this patient is glaucoma vs. some other retinal or neurological disease. The list of possible diseases would include any pathology that produces an asymmetric loss of neuroretinal rim tissue, a corresponding retinal nerve fiber layer defect and an associated visual field defect.
The following diseases share some of the clinical signs and symptoms of glaucoma.
- Retinal detachment
-
Pituitary tumors and other neurological conditions
-
Ischemic optic neuropathy
-
Vascular disease
-
Drusen of the optic disc
-
Congenital disc anomalies
Retinal detachments and drusen of the optic disc would be easily identified during routine ophthalmoscopy. Pituitary tumors producing morphologic changes in the optic nerve should be associated with characteristic bitemporal visual field defects. The patient did not have a history of vascular disease and no retinal vascular findings that would typically be associated with more advanced systemic vascular disease.
Ordering Diagnostic Tests
When additional clinical information is needed to complete the differential diagnostic process, diagnostic tests and procedures are ordered. The performance of these tests and procedures leads to the completion of the differential diagnostic process while simultaneously initiating the treatment program.
Based on the clinical diagnosis of glaucoma, the following diagnostic tests and procedures were ordered at the conclusion of the eye examination.
- Refraction
- Retinal nerve fiber layer scan
- Visual field examination
- Gonioscopy
- Fundus photography
The decision to order and perform additional testing is totally based on the concept of medical necessity which can only be determined by the examining optometrist or ophthalmologist.
Refraction
- Measuring visual acuity is a method of evaluating functional vision loss
- Advanced glaucoma can produce central visual field defects which result in a loss of visual acuity
- This patient had normal visual acuity in each eye
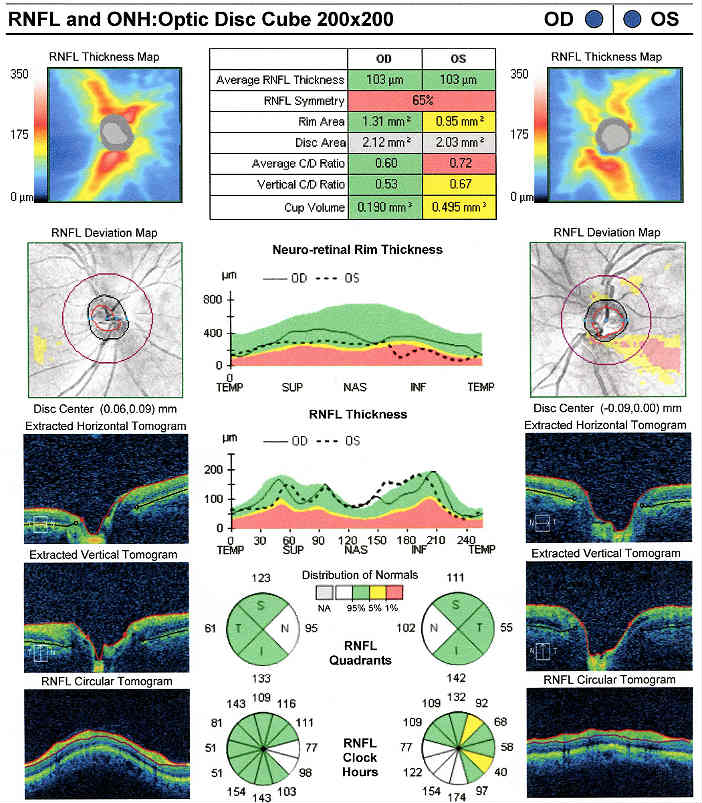
Retinal Laser Scan — Nerve Fiber Layer
- Measuring the average retinal nerve fiber layer thickness provides an overall assessment of retinal health
- The procedure can be accomplished by using the Cirrus OCT manufactured by Carl Zeiss Meditec
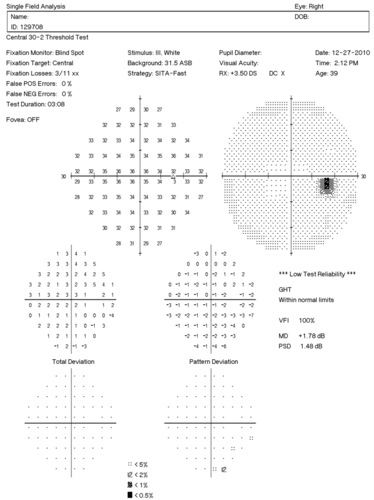
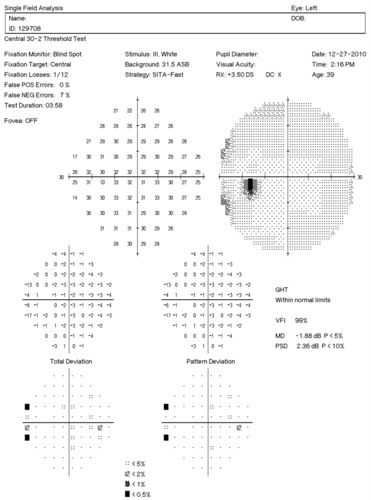
Visual Field Examination
- Automated threshold perimeters measure the visual field by plotting the threshold luminance value of the patient in various locations in the visual field
- The luminance of the light stimulus is represented by non-specific units of measurement called decibels (dB)
Glaucoma produces several changes in the visual field, and one of these changes occurs as a widespread, non-descript loss of retinal sensitivity. In Tranquair’s “Hill of Vision” concept, loss of retinal sensitivity represents a reduction in the height of the hill. The diffuse loss of retinal sensitivity should be considered highly diagnostic of glaucoma when it is asymmetric and correlates with asymmetric changes in intraocular pressure or disc appearance. In most cases, the loss of sensitivity occurs in characteristic patterns and locations (e.g., nasal step, arcuate scotoma, paracentral scotoma) that often correlate with changes in the optic nerve and/or retinal nerve fiber layer.
Automated threshold perimeters characterize specific parameters of the overall visual field status by the use of numbers called Global Indices. Two of the indices, Mean Deviation (MD) and Pattern Standard Deviation (PSD), express the raw data generated by the instrument.
A visual field defect can be classified as mild, moderate or advanced based upon an abnormal Mean Deviation.
- Mild visual field defect = 0 through -5.99 dB
- Moderate visual field defect = -6.00 dB through -11.99 dB
- Advanced visual field defect = anything above -12.00 dB
As represented by the Mean Deviation of +1.78 dB, the visual field of the right eye demonstrated no loss of retinal sensitivity.
As represented by the Mean Deviation of -1.88 dB, the visual field of the left eye demonstrated a mild loss of retinal sensitivity.
- Two paracentral scotomas were found in the temporal field of the left eye. Paracentral scotomas are visual field defects characterized by small, isolated areas of reduced retinal sensitivity. They are typically centered just beyond the central 10 degrees of fixation but usually within 20 degrees of fixation. Paracentral scotomas occur in about 70% of patients with early glaucomatous damage to the visual system.
The Pattern Standard Deviation is a measure of focal loss of visual field taking into account any generalized depression in the hill of vision. Some feel it is more diagnostic than the Mean Deviation in demonstrating glaucomatous field loss and the higher the PSD, the more likely the patient has glaucoma. Others feel the high degree of data manipulation contaminates the PSD value. In this patient, the PSD is significantly higher in the left eye and clinically significant asymmetry is diagnostic for glaucoma. Even though the threshold values are slightly lower in the nasal step area of the left eye, there is no definitive pattern defect in this patient that corresponds with the inferior optic nerve changes. Lack of complete agreement between structural (RNFL) and functional (VF) is a common problem in glaucoma, especially early glaucoma.
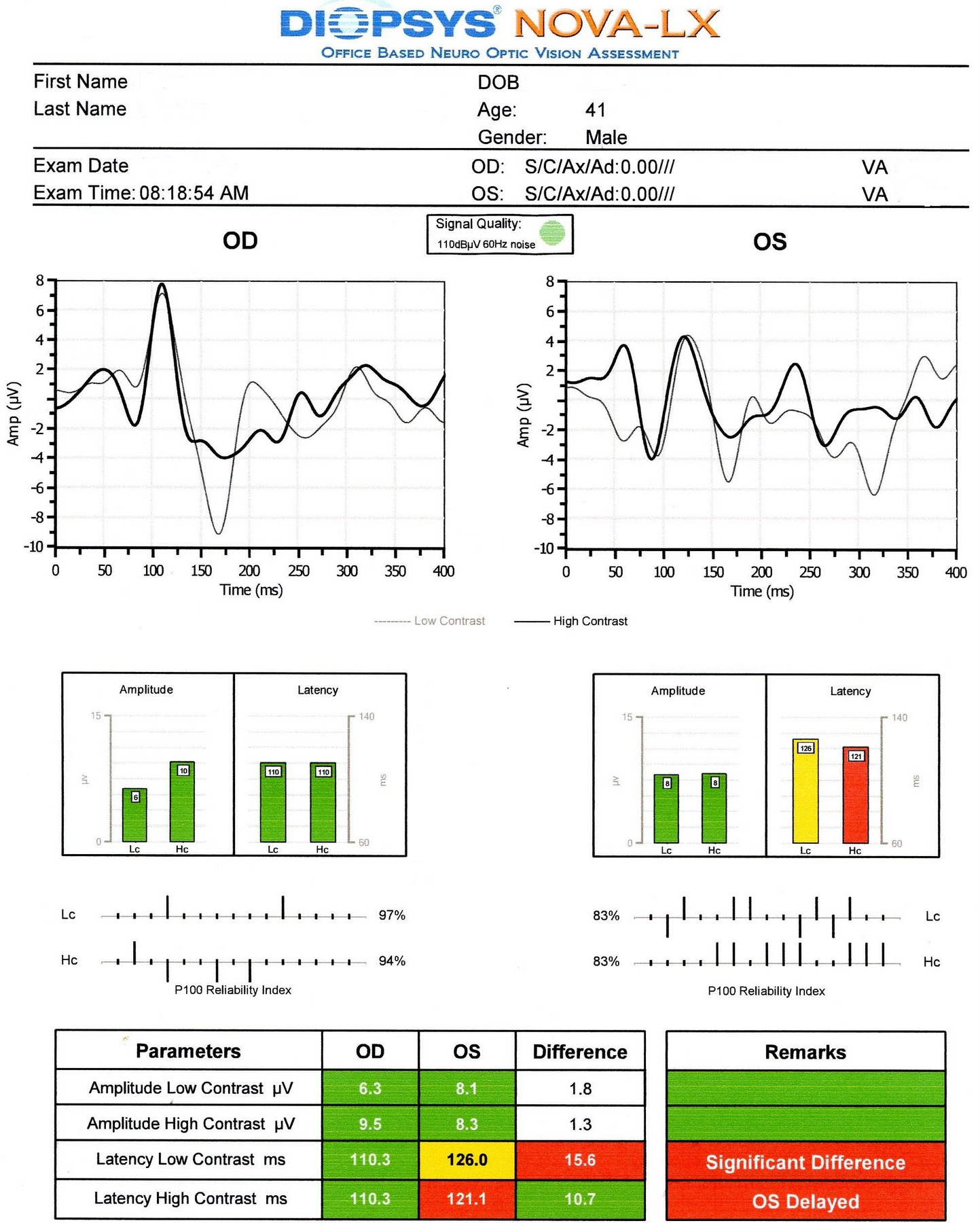
Visual Evoked Potential
Measuring the conduction speed and magnitude of the neural response from the eye to the cortex can be helpful in assessing early changes in glaucoma. Although not diagnostic of glaucoma, abnormal VEP test results in addition to other abnormal clinical findings can assist in making the difficult diagnosis of early glaucomatous damage.
- Visual evoked potential testing evaluates the integrity of the afferent visual sensory system
- Objective electric sign of visual pathway function
- Abnormal waveform shapes and delayed peak latencies helps to identify pathologies ranging from the eye through the brain to the primary visual cortex
- The procedure can be accomplished with the NOVA-LX VEP Testing Device manufactured by DIOPSYS
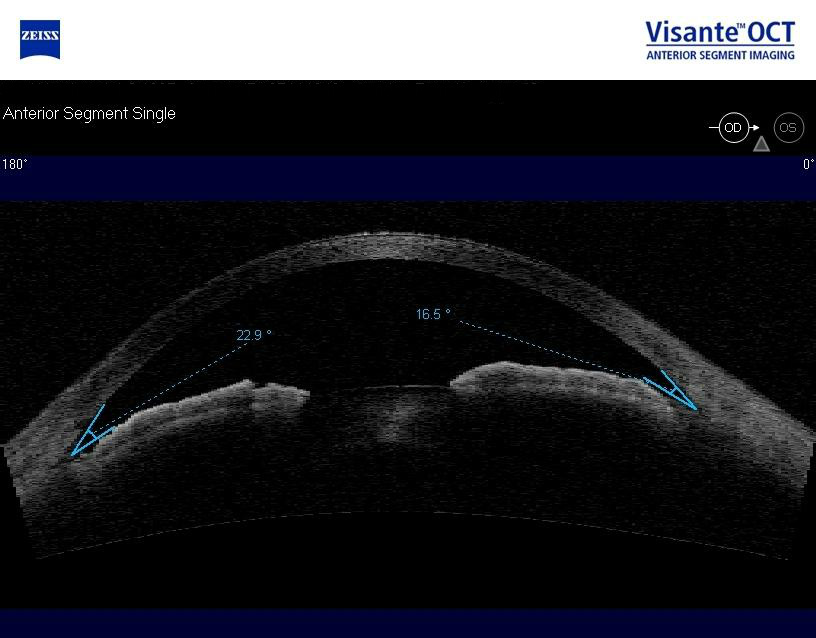
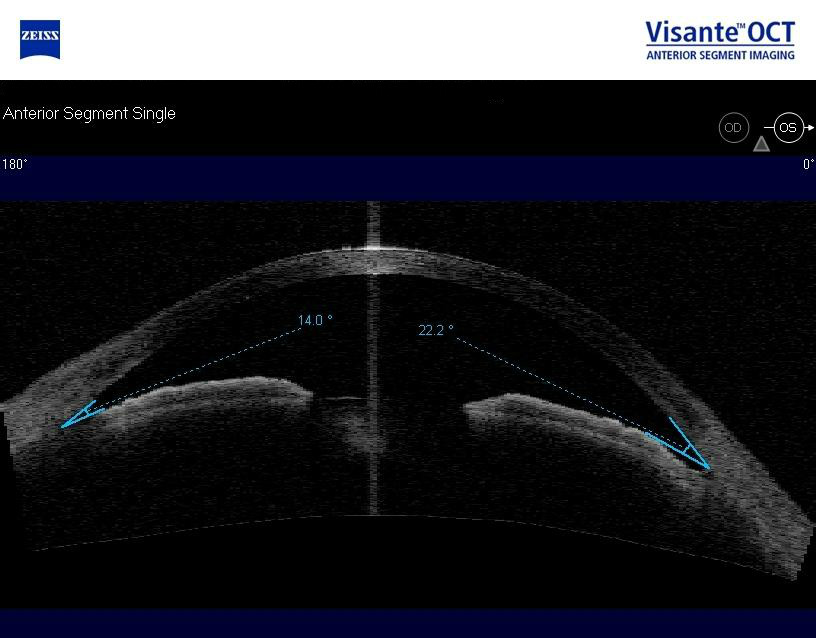
Anterior Segment Imaging
Imaging was performed and revealed completely open angles and a flat iris approach.
 |
 |
Fundus Photography
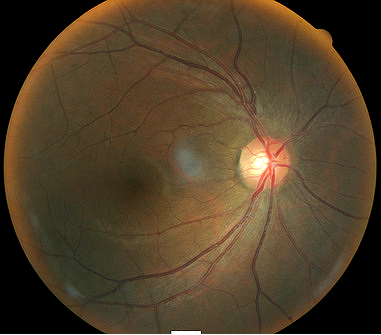
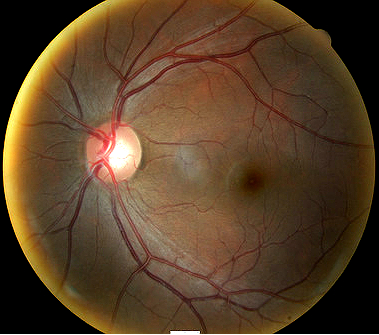
Fundus photography is a standard diagnostic test in all glaucoma patients. Photodocumentation of optic nerve and visible retinal nerve fiber layer changes are considered by many glaucoma specialists to be the single most important diagnostic test for monitoring glaucoma changes.
All of the diagnostic test results confirmed the initial diagnosis of elevated-tension open-angle glaucoma. Additional diagnostic tests for glaucoma such as serial tonometry, provocative glaucoma testing, or extended ophthalmoscopy were not medically necessary on this patient’s initial visit.
According to Current Procedural Terminology, when eye doctors perform ophthalmological examinations, the complexity of medical decision-making is not separated from the examining techniques used. As a guideline to assist eye doctors in enhancing their medical decision-making skills, consider that the complexity of medical decision-making involves three components.
The first component concerns the number of possible diagnoses and treatment options that must be considered. Modern glaucoma diagnosis involves the determination of structural damage to the eye and/or functional loss to the visual system. Once these assessments are made, the glaucoma must be classified by type.
The second component concerns the amount and complexity of medical records and diagnostic tests that have to be obtained, reviewed and analyzed. In addition to an eye examination, this visit required the review and analysis of a subjective refraction, a retinal laser scan, a threshold visual field examination, visual evoked potential testing and OCT imaging of the anterior chamber.
Third, the complexity of medical decision-making is affected by the risk of significant complications and/or morbidity associated with advanced glaucoma and the risks involved in any treatment options. This patient’s glaucoma was classified as a moderate problem where the risk of total vision loss without treatment was possible. In addition, because the treatment plan would involve chronic pharmaceutical treatment with the potential for ocular and systemic side effects, the pharmacology complications had to be considered when deciding on the initial treatment options.
Treatment Guidelines
To begin the treatment of glaucoma, intraocular pressure must be lowered and an initial target pressure range must be established. A general guideline is to base the initial pressure range on the category of damage to the visual system.
- Mild damage needs a 20-30% reduction in pre-treatment intraocular pressure as the initial target pressure range
- Moderate damage needs a 30-40% reduction in pre-treatment intraocular pressure as the inital target presure range
- Advanced damage needs a 40-50% reduction in pre-treatment intraocular pressure as the initial target pressure range
Due to the moderate glaucomatous damage in the left eye, a 30-40% reduction in the pre-treatment intraocular pressure was selected as the initial target pressure range. Target ranges are arbitrary and the goal is always to prevent additional vision loss, not achieve an arbitrary target IOP.
Treatment Program
Modern glaucoma therapy generally utilizes prostaglandins as the primary therapy. These medications have the greatest efficacy; and, the first goal of treating glaucoma is to achieve the lowest possible intraocular pressure on a single agent. Monotherapy with prostaglandins improves patient compliance, may decrease costs, and improves the safety profile and tolerability of using topical medications. These drugs reduce intraocular pressure by increasing uveoscleral outflow. Intraocular pressure reduction averages almost 30%.
Conjunctival hyperemia is the most common and, from a patient standpoint, the most problematic side effect of prostaglandin therapy. Other ocular side effects of prostaglandins include potential changes in iris color after several months of use. In these patients, the drug produces an increase in the amount of iris stromal pigmentation. Over time, eyelashes can lengthen, thicken, and darken – a side effect known as hypertrichosis.
The only serious ocular side effect is a 3% increased risk of developing cystoid macular edema. A history of uveitis is a relative contraindication, as prostaglandins produce an increased risk of reactivating the inflammation. The systemic side effects of topical prostaglandins are minimal.
Avoiding medicolegal liability if complications arise is an important part of modern ophthalmic practice. This avoidance is best accomplished by making sure that you have obtained the patient’s informed consent to treat their glaucoma. Once you have made the patient fully aware that using topical glaucoma medicine involves the risks of side effects, you can proceed with the treatment plan.
After obtaining the patient’s informed consent to treat his glaucoma, Lumigan ophthalmic solution (Allergan) was prescribed 1 drop at bedtime in both eyes.
A target pressure range of 17-20 mm Hg was established for the left eye.
Next visit scheduled for 3 weeks.
Discussion
This case demonstrates several key aspects of glaucoma diagnosis and treatment.
- First, despite the moderate damage to the left eye, there was no ocular emergency and no need for a referral to a glaucoma sub-specialist at this time. This patient had been functioning with glaucoma for at least a year. Another few days or weeks without treatment would probably not change the course of the disease.
- Second, this patient’s clinical history supports the modern understanding of the pathophysiology of glaucoma. We know that glaucoma is an optic neuropathy. Structural damage to the retinal nerve fiber layer is usually the first milestone in the natural history of glaucoma. The loss of retinal nerve fibers is closely followed by changes in the appearance of the optic disc. Functional loss, which is documented by abnormal visual field testing, abnormal visual evoked potential testing or loss of visual acuity, usually occurs later in the natural history of the disease.
- This case demonstrates how abnormalities found in VEP testing may precede measurable visual field defects.
On initial examination, the damage to the optic disc, the VEP abnormalities, and the loss of visual field was moderate in the left eye. The right eye, while having no measurable fallout of the retinal nerve fiber layer, had a large optic cup and mild overall depression of the visual field.
Based on the patient history, the nature of the presenting problem, and my own clinical judgement this patient needed an evaluation of the complete visual system.
- Perform the eye examination that is medically necessary
- Provide the diagnostic tests or services that are medically necessary
- Properly document the services provided
- Code from the documentation
- Report the services to the payor
Modifier 59
This modifier is used to identify a distinct procedural service for the same patient, on the same day, by the same provider. Documentation must support one of the following not ordinarily encountered or performed on the same day by the same doctor. A different diagnosis code is not necessary to use the -59 modifier.
- Different procedure or surgery
- Different session or patient encounter
- Separate incision or excision
- Separate lesion
According to the Centers for Medicare and Medicaid Services, modifier -59 should not be used to bypass a National Correct Coding Initiative edit. In this case, retinal laser scanning was necessary to document the glaucoma-induced nerve fiber layer defect and anterior segment imaging was needed to evaluate the anterior chamber angle.
| Diagnosis Code | Procedure Code | Modifier | Quantity | Payor | Amount Allowed |
| H40.1132 - Open-angle glaucoma, bilateral, moderate stage | 92014 - Medical eye examination | 1 | Medicare | 101.46 | |
| H40.1132 - Open-angle glaucoma, bilateral, moderate stage | 92015 - Refraction | 1 | Medicare | 42.94 | |
| H40.1132 - Open-angle glaucoma, bilateral, moderate stage | 92133 - Retinal laser scan | 1 | Medicare | 49.78 | |
| H40.1132 - Open-angle glaucoma, bilateral, moderate stage | 92083 - Visual field examination | 1 | Medicare | 73.34 | |
| H40.1132 - Open-angle glaucoma, bilateral, moderate stage | 92132 - Anterior segment imaging | 59 | 1 | Medicare | 34.73 |
| H40.1132 - Open-angle glaucoma, bilateral, moderate stage | 95930 - Visual evoked potential | 1 | Medicare | 106.02 | |
| Total | $408.37 |
H40.1132
Primary open-angle glaucoma,
bilateral, moderate stage
365.11
Open-angle glaucoma
92133
Retinal laser scan
92250
Fundus photography
95930
Visual evoked potential
92275
Electroretinography
92225
Extended ophthalmoscopy
76514
Corneal pachymetry
92132
Anterior segment imaging
92020
Gonioscopy
76513
Anterior segment ultrasound
92100
Serial tonometry




 Print | Share
Print | Share