Case Report ID: 31
Title:
Dry Age-Related Macular Degeneration (AMD) Revealed with Multi-Spectral Imaging
Category:
Macular Degeneration
Description:
Dry AMD is the most common type of macular degeneration and affects 90% of the people who have the condition. In the dry form, there is a breakdown or thinning of the layer of retinal pigment epithelial cells in the macula.
Age-related macular degeneration is a general term for any type of acquired maculopahy that affects people over 50-years-old. The most common type of age-related macular degeneration (AMD) is an atrophic form of the disease known as dry age-related macular degeneration.
Dry age-related macular degeneration (ARMD) is characterized by the following clinical features:
- Gradual breakdown of retinal pigment epithelial cells in the macula
- Gradual blurring of central vision
- Appearance, enlargement or coalescense of retinal drusen in the macula
- In advanced stages, development or progression of geographic atrophy
Case Report
- A 69-year-old white woman presented with a chief complaint of decreased vision the right eye
- Case history described a subjective decrease in vision over the past several years secondary to age-related dry macular degeneration in the right eye
- The patient had not been examined six months earlier and wanted to determine if there was any progression of her vision loss in either eye
Conclusion
Gradual atrophy of retinal pigment epithelial cells with resultant accumulation of metabolic debris and loss of photoreceptor function is the main cause of vision loss in dry age-related macular degeneration. Progression of atrophy like that seen in this patient is the culmination of prolonged, progressive changes in the retina. The most advanced presentation of this progressive atrophy can result in total loss of central vision known as geographic atrophy.
History of Present Illness
- Associated signs and symptoms: none
- Location: vision is worse in the right eye
- Duration: past one year
- Quality: n/a
- Context: decreased vision is more noticeable when reading
- Severity: mild decrease in vision
- Timing: vision seems to be getting worse over time
- Modifiers: none
Review of Systems
The patient reported that she was in good health and taking no medications.
Past, Family and Social History
Non-contributory
Best Corrected Distance Visual Acuity
- 20/50 in the right eye
- 20/25 in the left eye
Normal Examination Findings
- Mental status
- General medical observation
- Pupils
- Gross visual fields
- Basic sensorimotor examination
- External examination
- Adnexal examination
- External ocular examination with biomicroscopy
Intraocular Pressure Measurements
- 15 mm Hg in the right eye
- 16 mm Hg in the left eye
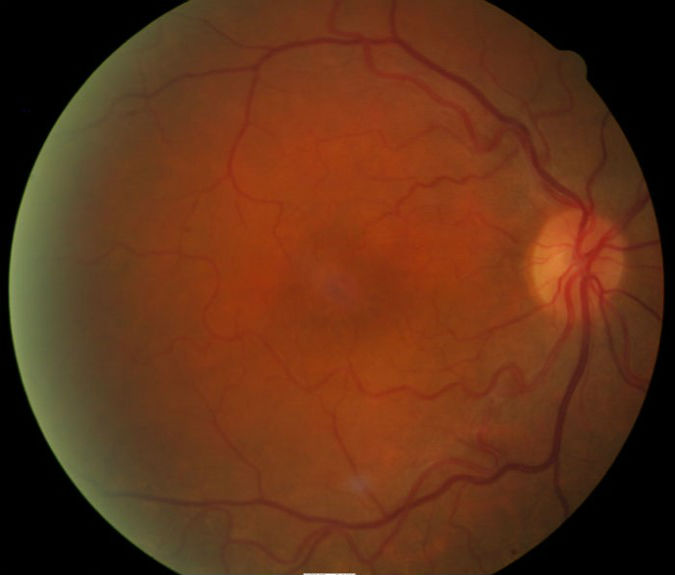
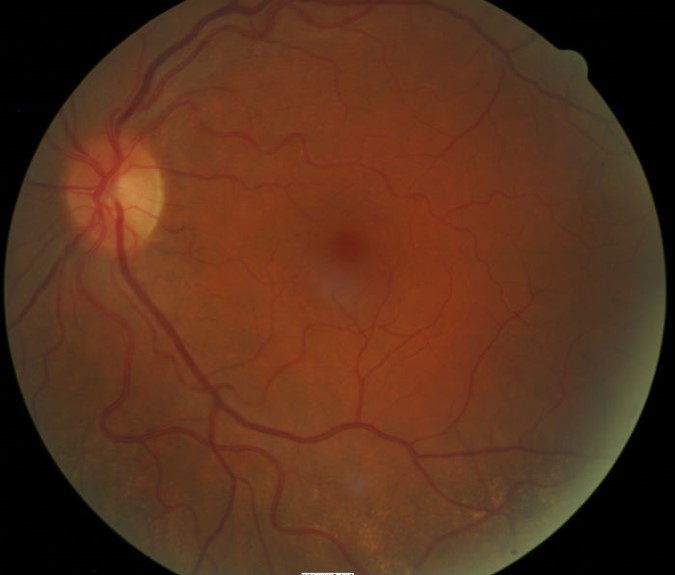
Ophthalmoscopy
- Focal degeneration at the macula, right eye
- Normal macular appearance, left eye
Clinical Diagnosis
The clinical diagnosis is a determination based on the knowledge obtained from the patient’s medical history and from the results of the eye examination alone, without the benefit of diagnostic tests or procedures.
The patient’s clinical diagnosis is dry age-related macular degeneration based on the following clinical findings:
- Focal atrophy of retinal pigment cells in the right macula
- Decreased visual acuity in the right eye
Treatment Plan
To gather the information required to treat macular degeneration, a diagnostic and treatment program is initiated.
- Determination of different types of diagnoses
- Selection of one or more treatment options
Several macular diseases have clinical signs that mimic the appearance of dry, age-related macular degeneration.
Differential Diagnoses
Familial Drusen
- Drusen usually outside macular region
- Drusen usually form circular pattern around inner posterior pole
- Rarely affects vision
Myopic Degeneration
- Only in high axial myopia
- Drusen rarely present
- Usually has associated peripheral retinal changes
Inflammatory Maculopathies
- Variable presentation of chorioretinal atrophy that may involve macula
- Associated inflammatory signs (vitreous cells)
Most patients with dry macular degeneration do not experience significant vision loss (90% less than 20/50). ARMD typically has an asymmetric presentation with the fellow eye showing clinical changes about one-third of the time within two years of the intial presentation in the first eye. There are multiple risk factors for ARMD but the most important are age, history of smoking, family history, poor vascular health, obesity, and nutritional factors. While the risk of progression to the most severe form of the disease (wet or exudative macular degeneration) is very low, monitoring for potential conversion to the wet form is the highest clinical concern.
Ordering Diagnostic Tests
The following diagnostic tests can help determine the severity of dry age-related macular degeneration and help monitor progression.
- Refraction
- 10-2 threshold visual field examination
- Retinal laser scan with spectral-domain OCT
- Multi-spectral imaging (MSI)
- Extended color vision examination
- Electroretinogram
- Dark adaptation testing
Some authorities also recommend additional testing including genetic testing and macular pigment density testing, both discussed later.
Refraction
- Measuring visual acuity is a method of evaluating functional vision loss
- Age-related macular degeneration can produce central visual field defects which result in a loss of visual acuity
- There was no improvement in the right eye’s visual acuity after a subjective refraction was performed or by pinhole testing
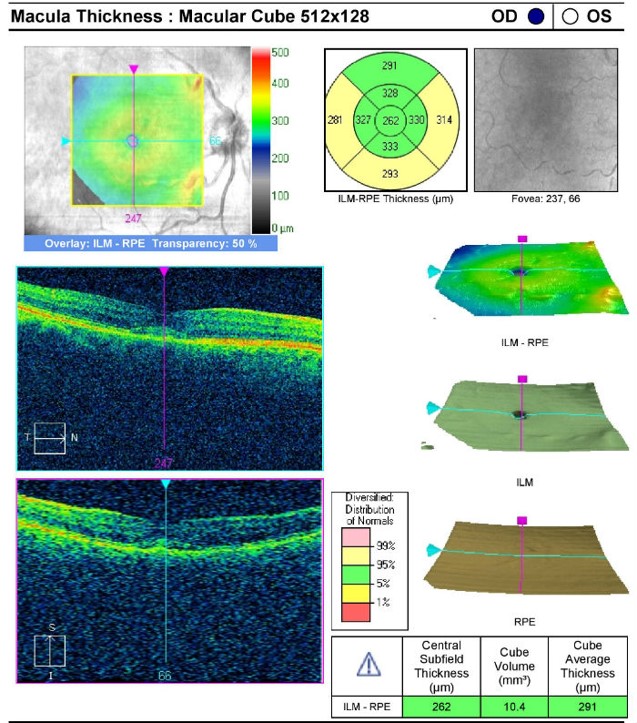
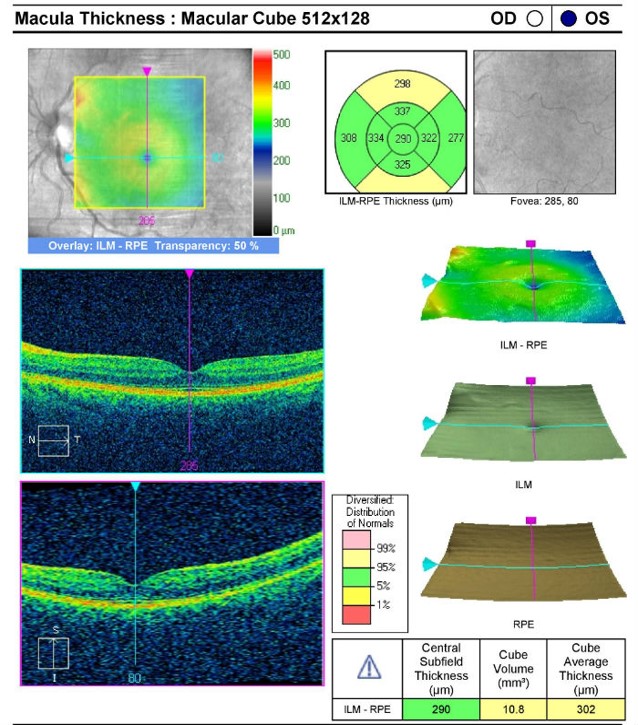
Retinal Laser Scan — Macula
- Measuring the thickness of the macula along with a detailed analysis of retinal structure provides an overall assessment of retinal health
- The procedure can be accomplished by using the Cirrus OCT manufactured by Carl Zeiss Meditec
 |
Retinal Laser Scan — Right Eye
|
|
Retinal Laser Scan — Left Eye
|
 |
|
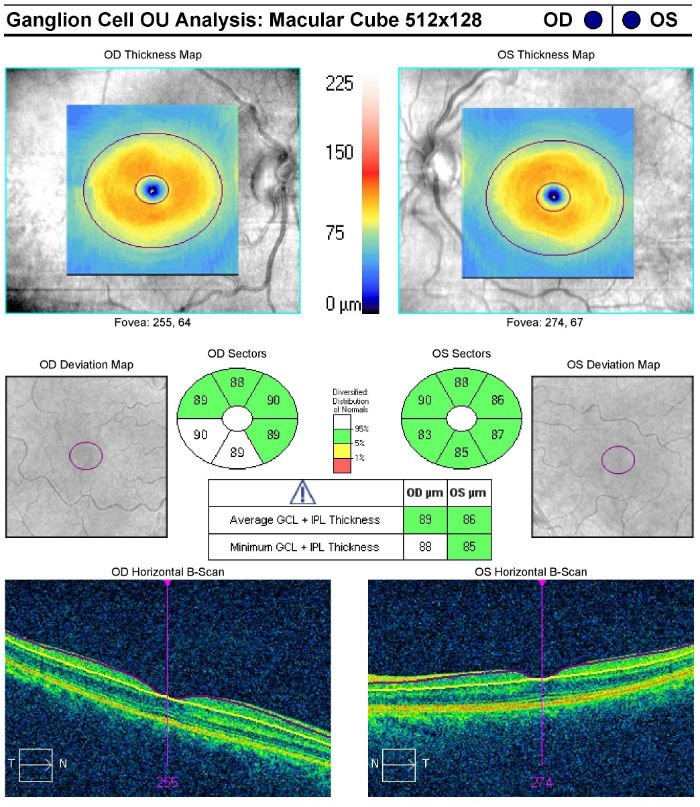 |
Ganglion Cell Analysis
|
Visual Field Examination
Automated threshold perimeters measure the visual field by plotting the threshold luminance value of the patient in various locations in the visual field. The luminance of the light stimulus is represented by non-specific units of measurement called decibels (dB).
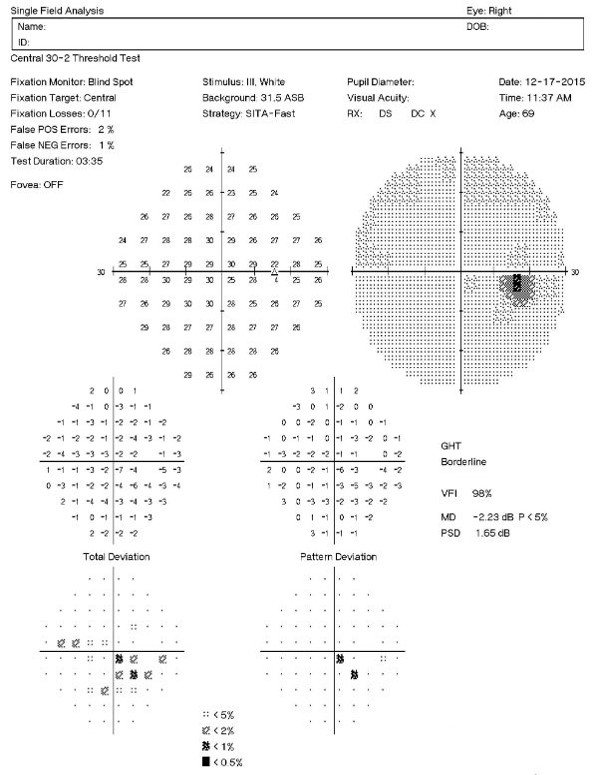 |
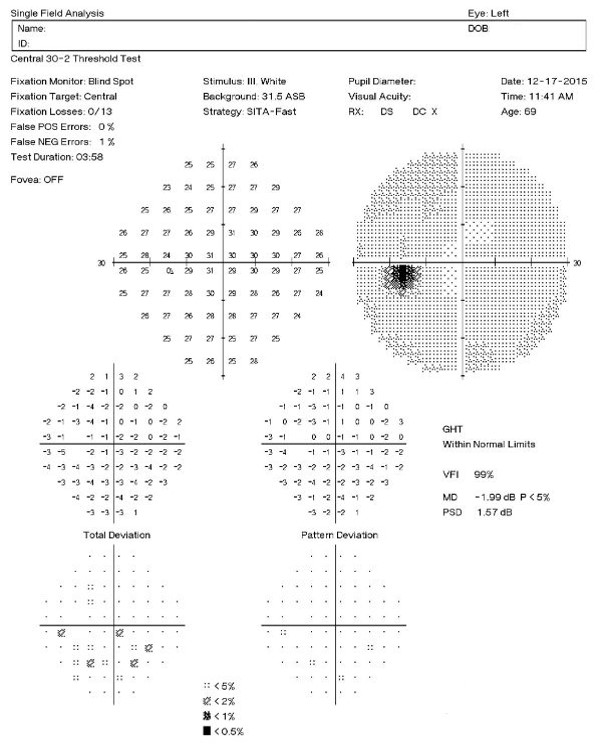 |
Clinically significant macular degeneration can produce a central scotoma in the visual field — even subtle macular degeneration can cause a decrease in the threshold values in the central points that loosely correlate to an expected degree of Snellen acuity.
- 32+dB — expect normal Snellen acuity
- High teens to low thirties — one or two lines loss
- Mid to high teens — three to four lines loss
- Low to mid teens — five to eight lines loss
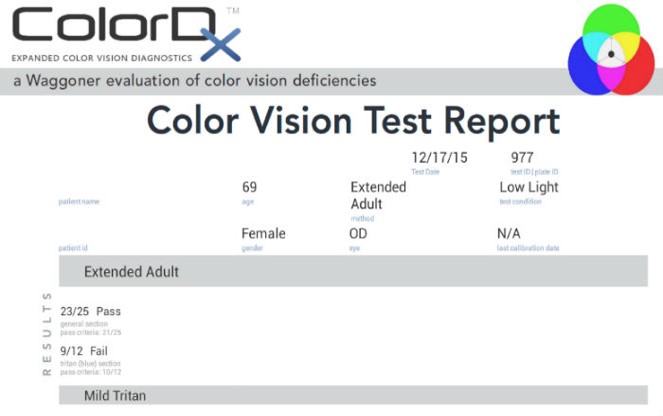
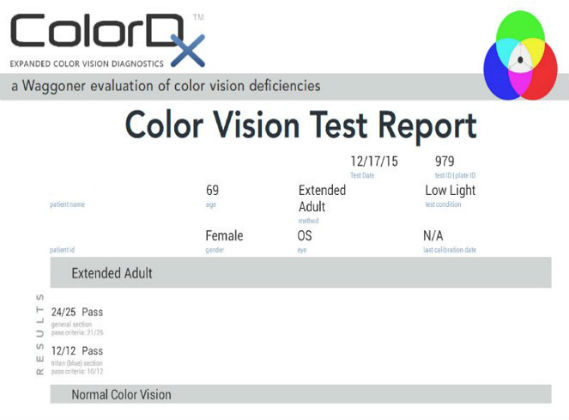
Color Vision Examination
An extended color vision examination measures the combined function of the retina, the optic nerve and the intracranial visual pathway and is used clinically to detect or monitor color vision loss due to disease at any of these locations.
In contrast to genetic color vision defects, which are always bilateral, acquired color vision defects can be monocular or different in each eye. Structural damage may occur at any of the following anatomical depths:
- Prereceptor (ocular media absorption properties)
- Receptor cells (red cones, blue cones, green cones and their ganglion cells)
- Postreceptor (retinocortical neural pathways in the brain to the visual cortex)
Multi-Spectral Imaging
- MSI technology captures image data at specific frequencies across the electromagnetic spectrum
- The Annidis RHA uses narrow band light emitting diodes to create a series of non-invasive en face spectral images throughout the retina and choroid
Monochromatic Light Sources on the Annidis RHA
- Green — Superficial structures highlighted (epiretinal membranes, vitreo-macular traction, cysts, folds macular holes)
- Yellow, amber, reds — Mid-retinal structures highlighted (diabetic retinopathy, neovascular membranes, drusen, exudates)
- Deep reds, infrareds — Deep retinal structures highlighted (dry AMD and retinal pigment epithelium structure, nevi, melanoma pigmentary anomalies)
- Fundus autofluoresence — Retinal pigment epithelium, subneurosensory space, outer retina (lipofuscin, atrophy)
- Yellow wavelength is tailored for detecting drusen and lipd deposits, retinal vasculature, oxy-hemoglobin absorption, and nerve fiber layer defects
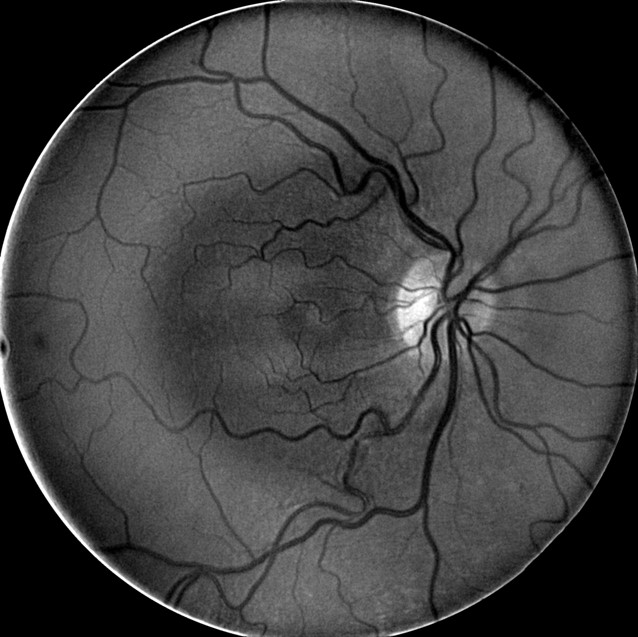 |
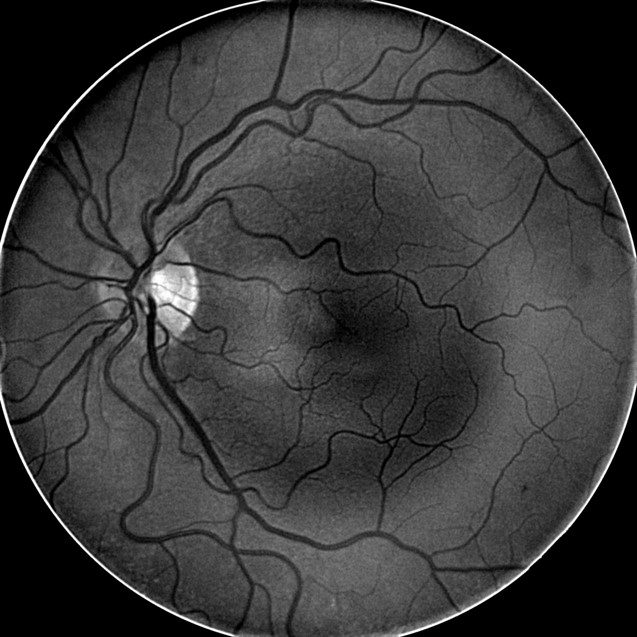 |
- Deep Red 3 wavelength images the outer retina and superficial choroid
- Best wavelength for imaging the retinal pigment epithelium
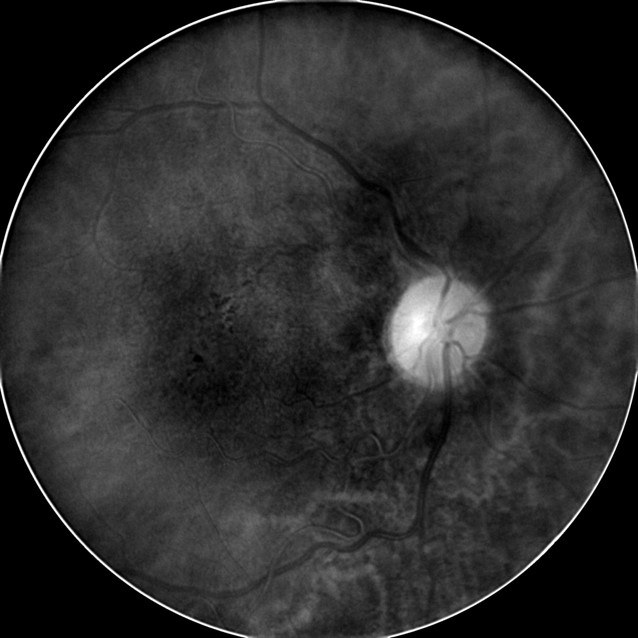 |
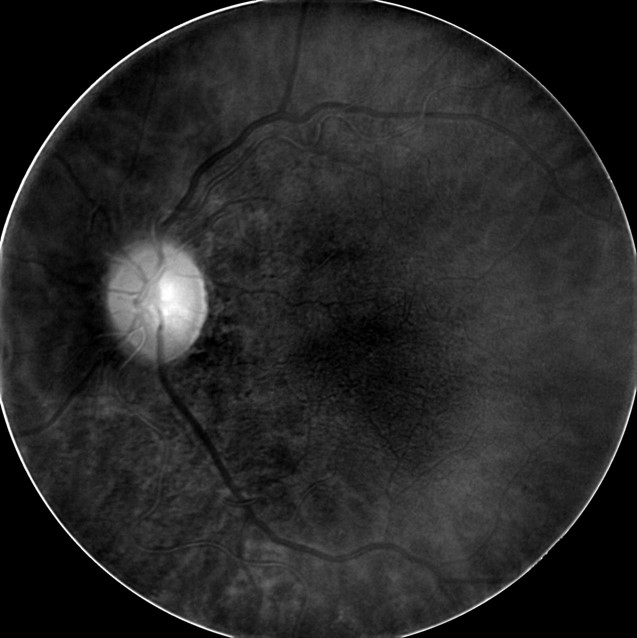 |
- MSI long wavelength fundus autofluorescence reveals damage to the right eye
- Hyperautofluorescence in the macula indicates abnormal lipofuscin accumulation
- Hypoautofluorescence in the macula indicates atrophy at the level of the RPE
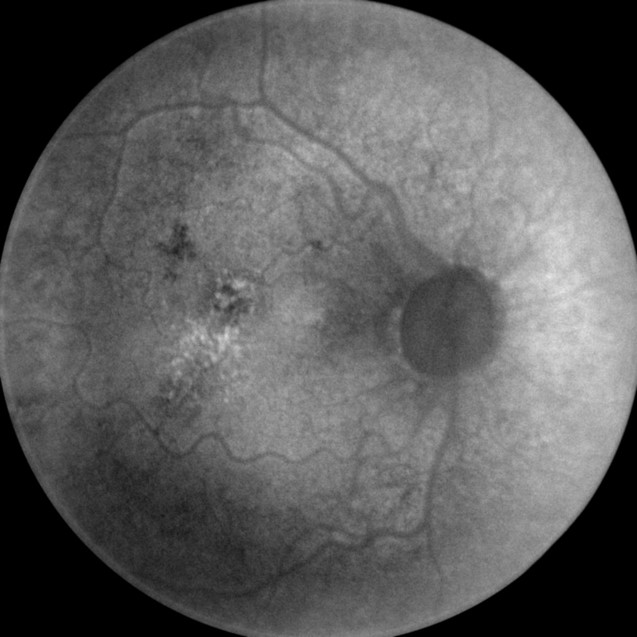 |
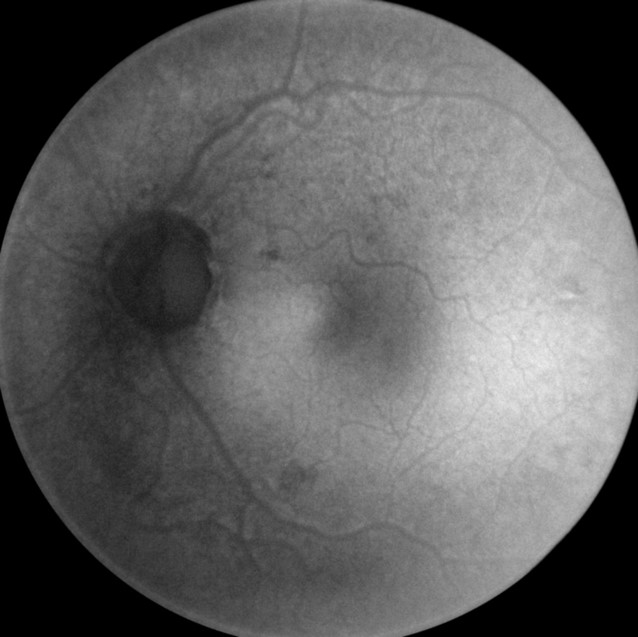 |
|
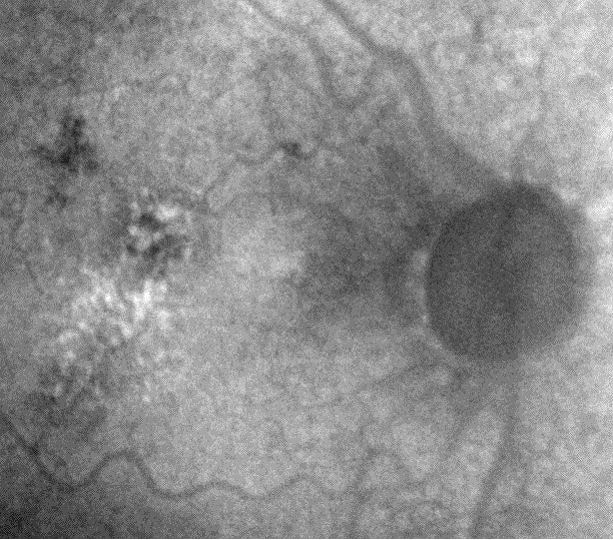 |
Fundus Autofluoresence Imaging
|
Physical Diagnosis
Because the central scotoma in the left eye agrees with measurable, observable structural damage to the left eye’s macula, the clinical diagnosis of age-related macular degeneration is confirmed.
Genetic Testing
While there is little doubt that genetics play a significant role in the pathogenesis of ARMD, current testing is still suspect for clinically significant sensitivity and specificity. At best, they may help indicate which patients who already have the disease may progress more rapidly. No currently available test can accurately predict who might develop ARMD.
Macular Pigment Density Tests
Once again, there is little doubt of the importance carotenoids play in maintaining macular health. Exactly how they relate to ARMD is still the subject of debate.
Of more concern is the lack of standards in evaluating macular pigment density, lack of knowledge regarding what is normal or abnormal in the individual patient presentation and how the measurement can or does relate the rate of vision decline. As explained in the summary on MPOD tests in Archives of Clinical and Experimental Ophthalmology 2011, clinicians should exercise caution in considering MPOD tests as an accurate predictor of ARMD onset or progression.
All of the diagnostic test results confirmed the initial diagnosis of subclinical macular degeneration in the right eye and mild-to-moderate macular degeneration in the left eye.
According to Current Procedural Terminology, when eye doctors perform ophthalmological examinations, the complexity of medical decision-making is not separated from the examining techniques used. As a guideline to assist eye doctors in enhancing their medical decision-making skills, consider that the complexity of medical decision-making involves three components.
The first component concerns the number of possible diagnoses and treatment options that must be considered. Determination of the diagnosis of macular degeneration involves the determination of structural damage to the retina with associated functional loss of vision.
The second component concerns the amount and complexity of medical records and diagnostic tests that have to be obtained, reviewed and analyzed. In addition to an eye examination, this visit required the review and analysis of a subjective refraction, a retinal laser scan, and a threshold visual field examination.
Third, the complexity of medical decision-making is affected by the risk of significant complications and/or morbidity associated with macular degeneration and the risks involved in any treatment options. This patient’s retinal condition was classified as a significant problem where the risk of significant central vision loss without treatment was possible. In addition, the treatment plan will involve continued monitoring of her retinal condition.
Treatment Guidelines
Dry macular degeneration is generally considered to be an irreversible process, even if detected early. Multiple studies, starting with the 1991 AREDS study, have shown variable benefit in the role of antioxidant therapy through macro-nutrient support. All the studies have random, somewhat arbitrary, and differing analysis of available nutrient supplements with highly variable claims of benefit in slowing progression of the disease. While the results could be considered somewhat less than exciting, nutritional support can still be considered the frontline therapy for at risk or confirmed ARMD patients. At this time, the most positive results support the use of the carotenoids lutein and zeazanthine as well as omega-3 fatty acids.
Patients with almost any stage of ARMD should be counseled regarding home monitoring of their disease. At a minimum, this should include paying close attention to the quality of their detailed vision. Amsler grids have been advocated for years. While very insensitive in detecting change, to the point of many authorities doubting their clinical value, they may help a small percentage of patients better detect qualitative changes in their vision.
There is no local (retinal) treatment for early, dry age-related macular degeneration so no referral to a retinal specialist is warranted.
Most importantly, those at risk or diagnosed with ARMD should be counseled regarding the deleterious effects of smoking and obesity.
Treatment Program
The patient was counseled regarding the nature of her disease and given an Amsler grid for home monitoring. It was recommended she begin nutritional supplementation in the the form of lutein 30mg/day, zeazanthine 30mg/day, and omega-3 supplements containing at least 2000mg/day of EPA/DHA.
Next visit scheduled for 6 months.
Discussion
This case demonstrates several key aspects of monitoring the current knowledge base in ocular disease. It is important to accurately follow patients with ARMD with the greatest concern in watching for conversion to the more serious wet or exudative form of the disease. While accurate detection of ARMD is possible with normal ophthalmoscopic examination, additional diagnostic testing can be very beneficial in confirming the diagnosis and monitoring for disease progression. As the changes with advancing disease are often suble, progression is best monitored by SD OCT technology with RPE change analysis and or autofluorescence capability. Although sometimes beneficial, visual field testing and Amsler grid monitoring are subject to extremely poor sensitivity in detecting change. Treatment of dry ARMD is at this time limited to lifestyle changes and supplementation with antioxidants.
An evaluation of the complete visual system was performed.
- Perform the eye examination that is medically necessary
- Provide the diagnostic tests or services that are medically necessary
- Properly document the services provided
- Code from the documentation
- Report the services to the payor
Physicians Quality Reporting System
PQRS Measure 14: Age-Related Macular Degeneration: Dilated Macular Examination – CPT code 2019F.
This measure applies to patients 50 years and older diagnosed with age-related macular degenertion. CPT code 2019F is reported when these patients receive a dilated macular examination. The measure should be reported on the date of the dilated examination and on all subsequent eye examinations during the next 12 months – even if a dilated macular examination was not performed on the follow-up examinations. Remember that you may be required to report this measure more than once since the reporting period covers 12 months.
PQRS Measure 40: Age-Related Macular Degeneration: Counseling on Antioxidant Supplement – CPT code 4177F
This measure applies to patients 50 years and older diagnosed with age-related macular degeneration. CPT code 4177F is reported when these patients receive counseling on antioxidant supplementation (e.g., per AREDS recommendations). The measure should be reported on the date of the initial diagnosis and on all subsequent eye examinations during the next twelve months – even if counseling was not performed on the follow-up examinations. Remember that you may be required to report this measure more than once since the reporting period covers 12 months.
PQRS Measure 130: Current Medications with Name, Dosage, Frequency and Route Documented – CPT code G8427.
Multiple Procedure Payment Reduction
Effective January 1, 2013, there is a small reduction in payment from Medicare if certain multiple procedures are billed on the same day. The fee for the technical component of the diagnostic test for the second and subsequent tests will be reduced by 20%. The second diagnostic test and subsequent tests should be reported with a -51 modifier. Professional services such as gonioscopy, extended ophthalmoscopy and provocative glaucoma testing are excluded from this policy. Visual evoked potential testing is excluded from this policy.
Modifier 51
This modifier is used to identify the secondary procedure or when multiple procedures are performed on the same day by the same provider. List the major primary procedure first and append the modifier to the subsequent procedure. The primary procedure is the one with the highest dollar value.
| Diagnosis Code | Procedure Code | Modifier | Quantity | Payor | Amount Allowed |
| H35.31 - Nonexudative senile macular degeneration of retina | 92014 - Medical eye examination | 1 | Medicare | 127.36 | |
| H35.31 - Nonexudative senile macular degeneration of retina | 92083 - Visual field examination | 51 | 1 | Medicare | 58.45 |
| H35.31 - Nonexudative senile macular degeneration of retina | 92250 - Fundus photography | 1 | Medicare | 80.05 | |
| H35.31 - Nonexudative senile macular degeneration of retina | 92283 - Extended color vision examination | 51 | 1 | Medicare | 51.28 |
| H35.31 - Nonexudative senile macular degeneration of retina | 4177F - Counseling on antioxidant supplement | Medicare | 0.00 | ||
| H35.31 - Nonexudative senile macular degeneration of retina | G8427 - Current medications documented | Medicare | 0.00 | ||
| Total | $317.14 |
H35.31
Nonexudative age-related
macular degenertion
362.51
Nonexudative senile macular degeneration of retina
92015
Refraction
92250
Fundus photography
92083
Visual field exam
92283
Color vision exam
92134
Retinal laser scan
92225
Extended ophthalmoscopy
92275
Electroretinography




 Print | Share
Print | Share