Case Report ID: 52
Title
Diabetes-Induced Retinal Ischemia
Category
Diabetic Retinopathy (3)
Description
Diabetic retinopathy can result in retinal ischemia due to capillary non-perfusion.
Diabetes is a disease in epidemic proportion in the United States. In 2012, it is estimated that approximately 26 million people in the United States are diabetic and another 79 million are pre-diabetic. Dramatic increases in disease prevalence are expected. In addition to the significant array of systemic complications associated with diabetes, ocular complications are common. Diabetic changes in the retina occur 60% of the time in diabetics presenting with more than a 10 year history of the disease. This increases to 80% at 15 years with 25% developing proliferative changes at this point.
The role of the primary eye care provider cannot be over-emphasized in the management of the diabetic patient. As the ocular findings are simply a manifestation of what is happening everywhere in the vascular system, primary care physicians and endocrinologists managing the systemic disease are always very interested in the patient’s ocular health. Timely communication regarding the ocular findings is good medicine.
Clinical Presentation
- 54-year-old black woman presents for her scheduled diabetic retinopathy surveillance examination
- She reports a subjective decrease in vision since her last visit six months earlier
Conclusion
Diabetics with mild to moderate retinal pathology can present with a wide array of symptoms from none at all to moderate vision loss. While diabetes can cause changes in refractive error, this is usually reserved only for patients with wildly uncontrolled blood sugar. Optometrists and ophthalmologists should be aware of all the ocular complications that can be associated with diabetes.
History of Present Illness
- Associated signs and Symptoms: reading vision worse
- Location: night vision is overall worse
- Duration: at least the past three months
- Quality: vision is worse in dimmer light
- Context: decreased vision is more noticeable when driving
- Severity: mild
- Timing: getting progressively worse
- Modifiers: none
Review of Systems
The patient reported diabetes diagnosed 22 years prior, hypertension and depression. She was taking Mavik (tranolalin) for her hypertension but was controlling her diabetes with diet and exercise. She did state she took diabetic medication in the past but none in the past five years. She did not know her last HbA1c but monitors her blood sugar every week or so. She reported it was “controlled” with most readings around 250mg/dL. The patient had a normal body habitus. All other medical history was unremarkable.
Past, Family and Social History
- Non-contributory
Best Corrected Distance Visual Acuity
- 20/30 in the right eye
- 20/25 in the left eye
Normal Examination Findings
- Mental status
- General medical observation
- External examination
- Adnexal examination
- External ocular examination with biomicroscopy
RAPDx Expanded Pupil Diagnostics
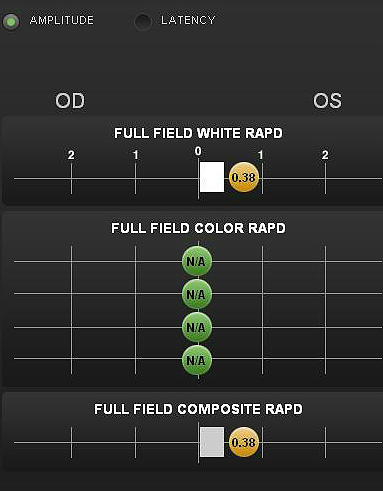
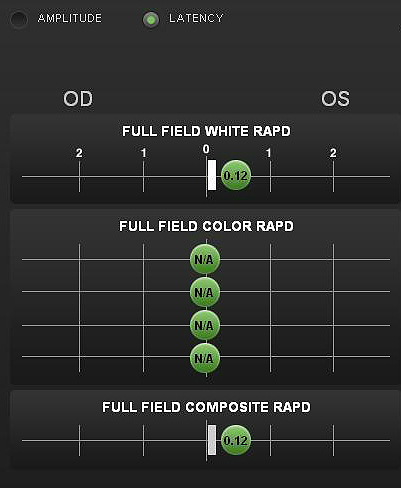
Pupillary reactivity testing using a RAPDx automated pupillographer was abnormal.
- A relative afferent pupillary defect was detected in the left eye
- Index-of-defect number was 0.38 for the amplitude of the pupillary response in the left eye
- Number is calculated by determining the log difference in amplitude between the pupillary responses
- Numbers above 0.30 are considered abnormal
- Higher the number, the more likely pathology is present in the neural light reflex pathway
Clinical Diagnosis
Clinical diagnoses are determined based on the knowledge obtained from the patient’s medical history and from the results of the eye examination alone, without the benefit of diagnostic tests or procedures.
The patient’s clinical diagnoses is mild nonproliferative diabetic retinopathy based on the following clinical findings:
- Appearance of the retina
- Patient’s medical and ocular history
Treatment Plan
To gather the clinical information required to manage the diabetic changes, a diagnostic and treatment program is initiated.
- Determination of different types of diagnoses
- Selection of one or more treatment options
Several macular diseases have clinical signs that mimic the appearance of diabetic retinopathy. Optometrists and ophthalmologists should keep in mind that retinal vascular presentations can be the result of systemic co-morbidities, especially diabetic and hypertensive complications.
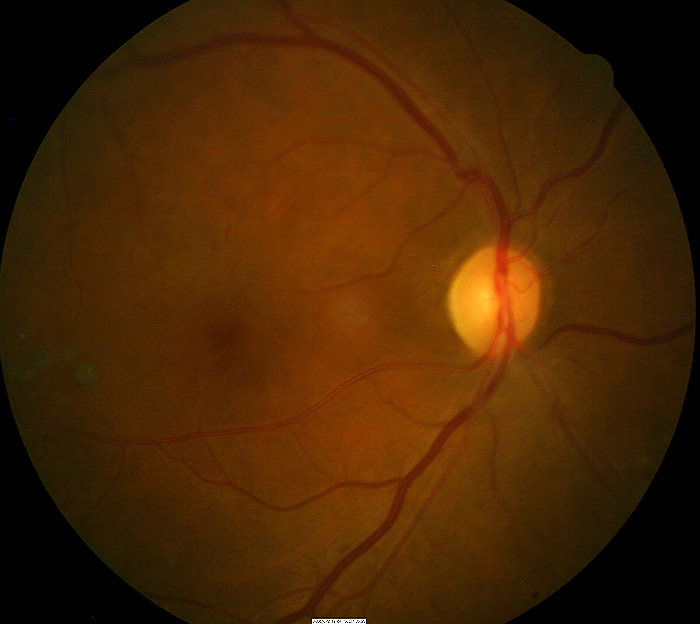
Most patients with diabetic retinopathy experience mild to no symptoms unless macular edema is present. Even then, mild macular edema can present with normal Snellen acuity. Ring like exudation, often referred to as circinate retinopathy, is often associated with areas of capillary non-perfusion and indicative of more advanced disease progression. Macular edema can occur at any stage of the disease, even in the absence of retinal hemorrhages and exudates.
The risk of progression to more advanced disease (pre-proliferative or proliferative) is variable but most influenced by the severity and control of the systemic diabetes and the duration of the diabetes. Conversion to proliferative disease and the appearance of macular edema are the two most significant concerns in monitoring the diabetic patient.
Ordering Diagnostic Tests
The following diagnostic tests can help determine the severity of the diabetic retinal changes and help to monitor progression:
- Subjective refraction
- Fundus photography
- Retinal laser scan
- Threshold visual field
- Color vision examination
- Electroretinography
The decision to order and perform additional testing is totally based on the concept of medical necessity which can only be determined by the examining optometrist or ophthalmologist.
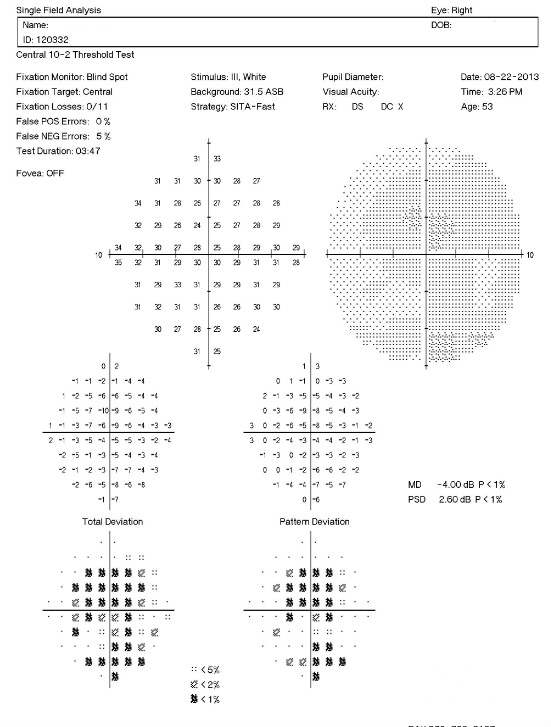
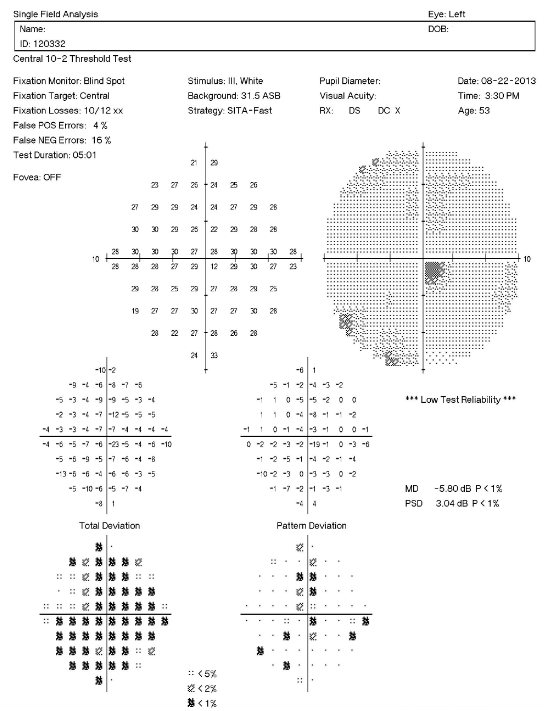
Visual Field Examnination
- Automated threshold perimeters measure the visual field by plotting the threshold luminance value of the patient in various locations in the visual field
- The luminance of the light stimulus is represented by non-specific units of measurement called decibels (dB)
- Glaucoma produces several changes in the visual field, and one of these changes occurs as a widespread, non-descript loss of retinal sensitivity
- In Tranquair’s “Hill of Vision” concept, loss of retinal sensitivity represents a reduction in the height of the hill
- The diffuse loss of retinal sensitivity should be considered highly diagnostic of glaucoma when it is asymmetric and correlates with asymmetric changes in intraocular pressure or disc appearance
- In most cases, the loss of sensitivity occurs in characteristic patterns and locations (e.g., nasal step, arcuate scotoma, paracentral scotoma) that often correlate with changes in the optic nerve and/or retinal nerve fiber layer.
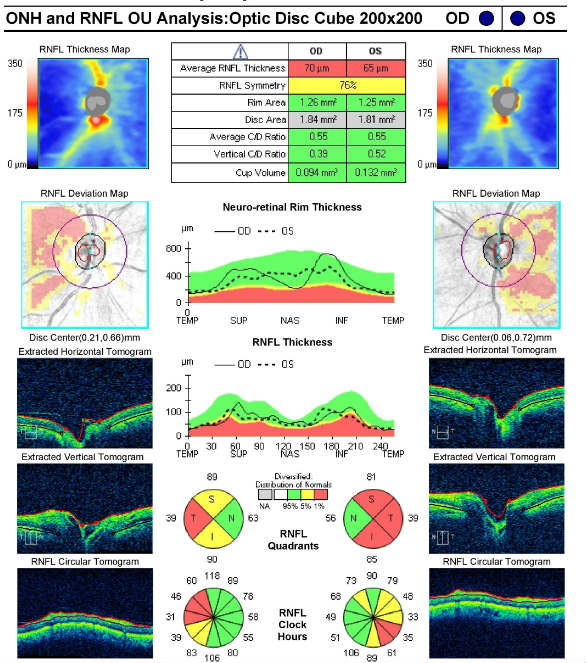
Retinal Laser Scan — Nerve Fiber Layer
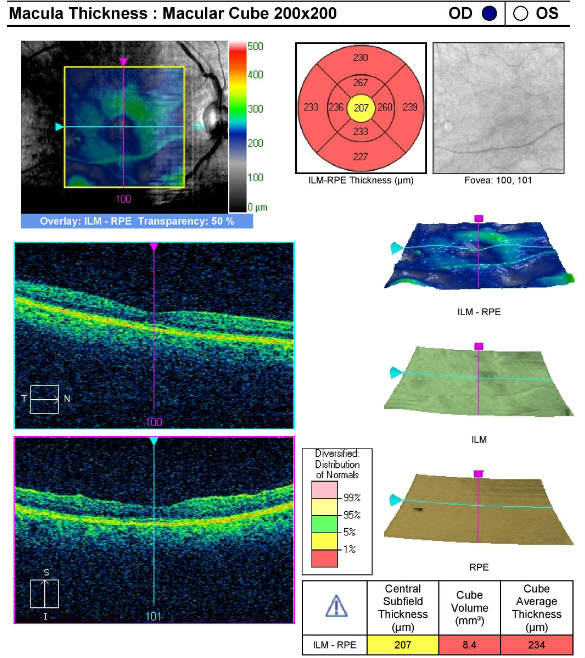
- Measuring retinal thickness and detailed evaluation of the retinal anatomy is a means to evaluate overall retinal health
- The procedure can be accomplished with the Cirrus OCT manufactured by Zeiss Meditec
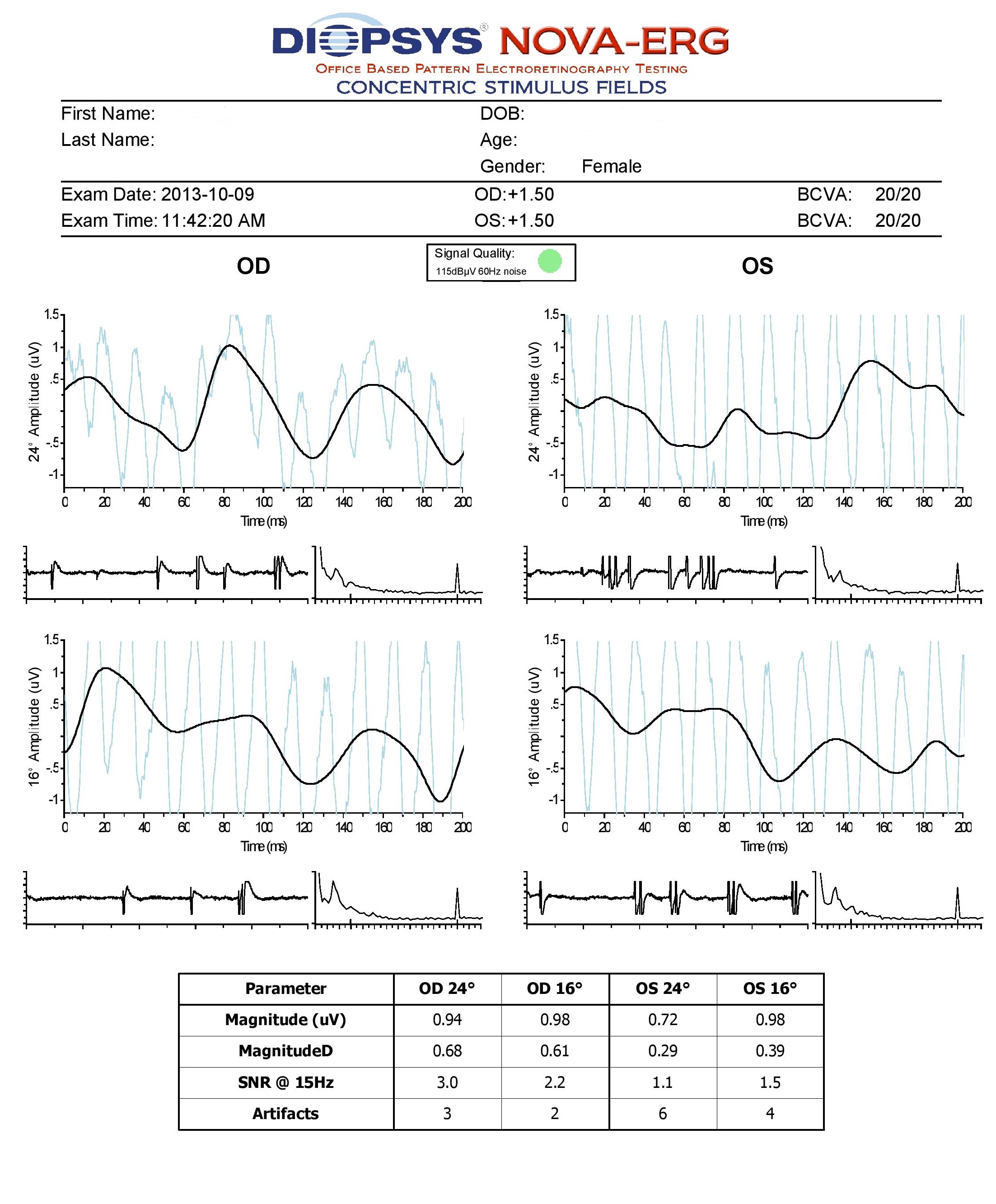
Electroretinography
- Electroretinography tesing evaluates the integrity of the retina
- The procedure can be accomplished using the NOVA-ERG Testing Device manufactured by DIOPSYS
- Specialized pattern electroretinogram (pERG) test uses a contrast-reversing pattern for the stimulus to produce information about ganglion cell function
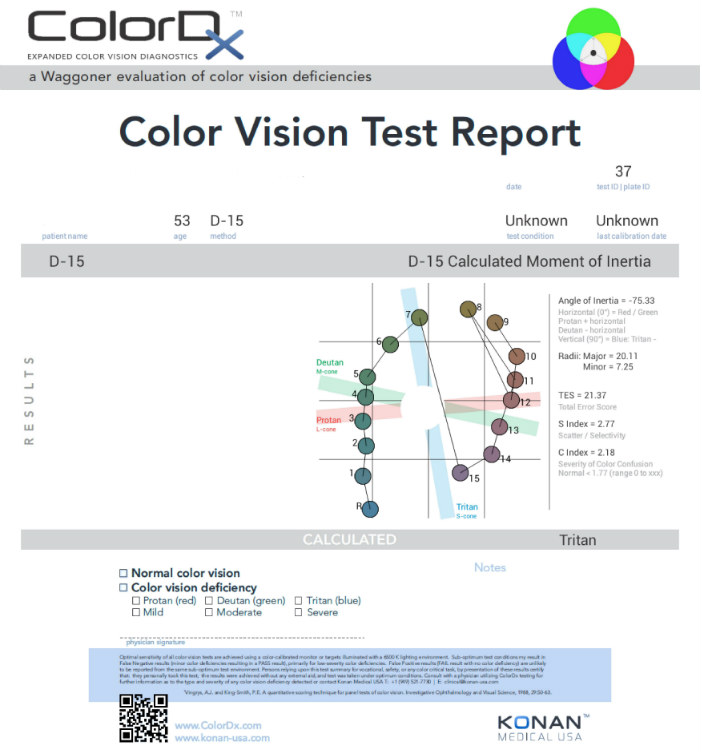
Color Vision Examination
An extended color vision examination measures the combined function of the retina, the optic nerve and the intracranial visual pathway and is used clinically to detect or monitor color vision loss due to disease at any of these locations. Extended color vision testing divides people into two groups.
- The first group consists of people with normal color vision and slight color deficiency
- The second group consists of people with moderate or severe color deficiency
One of the most common tests performed in clinical practice is the Farnsworth D15. When performed on Konan Medical’s ColorDx software , the test is presented as fifteen colored bars and one fixed bar on an Android tablet. The hue of each bar has been chosen so that the adjacent bars have approximately equal hue differences. When the bars are arranged in order, they form a hue circle. As a result, errors in hue discrimination can be made across the hue circle.
Patients with normal color vision usually make no error but may make one or two minor transposition errors, as do those with mild color vision deficiency. Patients with more advanced color deficiency make some or more of the following errors:
- They place colors that lie on the opposite side of the hue circle next to colors that lie on their confusion locus (e.g., diametrical errors)
- Two or more diametrical crossing errors indicates a “failed” test
- If diametrical crossing errors are made, the orientation of the crossing produces a diagnosis of protan, deutan, or tritan color vision deficiency
 |
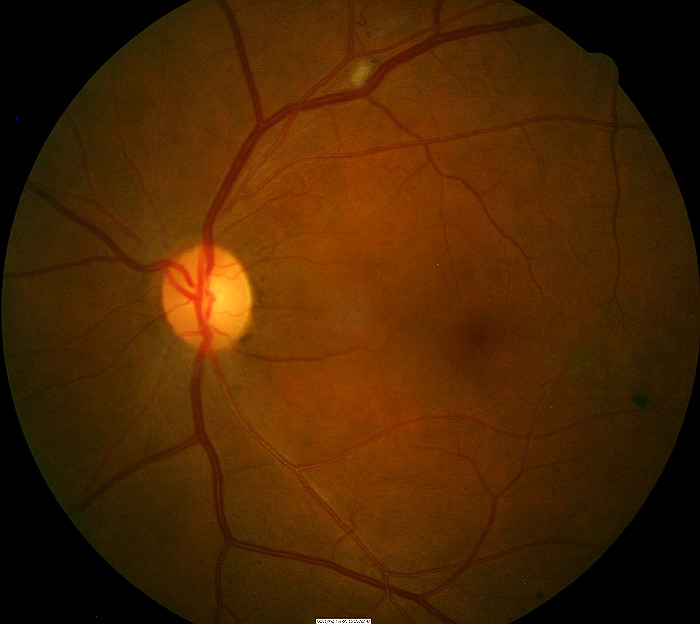
Right Eye
|
|
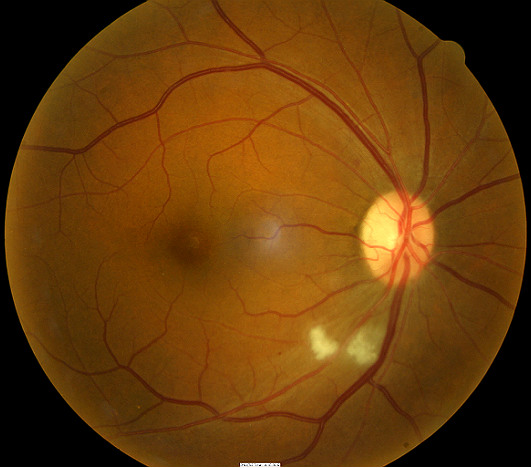
Left Eye
|
 |
All of the diagnostic test results confirmed the initial diagnosis of moderate, non-proliferative diabetic retinopathy with clinically significant macular edema.
According to Current Procedural Terminology, when eye doctors perform ophthalmological examinations, the complexity of medical decision-making is not separated from the examining techniques used. As a guideline to assist eye doctors in enhancing their medical decision-making skills, consider that the complexity of medical decision-making involves three components.
The first component concerns the number of possible diagnoses and treatment options that must be considered. Many vascular and some non-vascular conditions can manifest with retinal findings similar to diabetic retinopathy. These are listed in the Initial Diagnosis section.
The second component concerns the amount and complexity of medical records and diagnostic tests that have to be obtained, reviewed and analyzed. In addition to an eye examination, this visit required the review and analysis of a subjective refraction, a retinal laser scan, a threshold visual field examination and fundus photography.
Third, the complexity of medical decision-making is affected by the risk of significant complications and/or morbidity associated with diabetic retinopathy and the risks involved in any treatment options. This patient’s diabetic retinopathy was classified as a significant problem where the risk of total vision loss without treatment was significant and there was significant potential for other systemic co-morbidities. In addition, coordination of care are essential elements of patients presenting with sight threatening disorder of this nature.
Treatment Guidelines
Management of diabetic retinopathy is mostly influenced by the stage of the presenting disease. In most cases, diabetic retinal pathology has not progressed to the point where interventional treatment is required. Most will require timely monitoring of the ocular complications and coordination of care with the patient’s primary care physician and/or endocrinologist.
Specific to the diagnosis of diabetic retinopathy, the ETDRS study guidelines are still considered the preferred practice pattern for patients presenting with ocular complications from diabetes. While not reviewed here in detail, up to the point of either severe pre-proliferative or proliferative findings or clinically significant macular edema, photodocumentation, imaging and careful monitoring of the condition is adequate. The more severe the presentation, the more often progress evaluations should occur. If the patient has proliferative disease, at significant risk for developing proliferative disease, or, has clinically significant macular edema per ETDRS guidelines, the patient should be referred to a retinal specialist for consideration of treatment. Intravitreal VEGF drugs have become the most common initial treatment modality but focal and panretinal photocoagulation are still necessary components of treating proliferative disease.
Treatment Program
The patient was counseled strongly regarding her systemic disease and the resultant ocular complications. The retinal presentation was documented by both photography and imaging.
Evaluation of the entire ocular system with consideration of the visual field results and clinical presentation, it was felt the night vision complaints were secondary to the damage done to her retina from the diabetes. The patient was counseled regarding this finding and that no additional laser surgery or intraocular injections were necessary at this time. The patient was asked to return for additional evaluation in four months.
- Perform the eye examination that is medically necessary
- Provide the diagnostic tests or services that are medically necessary
- Properly document the services provided
- Code from the documentation
- Report the services to the payor
Physicians Quality Reporting System
PQRS Measure 19: Diabetic Retinopathy: Findings of dilated macular or fundus exam communicated with the physician responsible for managing ongoing diabetic care – CPT code 5010F + G8397
This measure applies to patients 18 years and older diagnosed with diabetic retinopathy. CPT codes 5010F and G8397 are reported together when these patients receive a dilated macular or fundus examination.
The measure should be reported on the date of the dilated examination and on all subsequent eye examinations during the next 12 months – even if a dilated macular or fundus examination was not performed on the follow-up examinations. If a dilated examination was not performed, use CPT code G8398. Remember that you may be required to report this measure more than once since the reporting period covers 12 months.
PQRS Measure 226: Patient screened for tobacco use and identified as a non-user of tobacco – CPT code 1036F
This measure applies to patients 18 years and older. The measure should be reported on the day of the examination and can be used with any diagnosis code.
PQRS Measure 130: Current Medicatons with Name, Dosage, Frequency and Route Documented – CPT code G8427
This measure applies to patients 18 years and older. The measure should be reported on the day of the examination and can be used with any diagnosis code.
Modifier 59
This modifier is used to identify a distinct procedural service for the same patient, on the same day, by the same provider. Documentation must support one of the following not ordinarily encountered or performed on the same day by the same doctor. A different diagnosis code is not necessary to use the -59 modifier.
- Different procedure or surgery
- Different session or patient encounter
- Separate incision or excision
- Separate lesion
According to the Centers for Medicare and Medicaid Services, modifier -59 should not be used to bypass a National Correct Coding Initiative edit. In this case, fundus photography was necessary to document the retinal hemorrhaging and a retinal laser scan was needed to identify or exclude diabetic macular edema.
Multiple Procedure Payment Reduction
Effective January 1, 2013, there is a small reduction in payment from Medicare if certain multiple procedures are billed on the same day. The fee for the technical component of the diagnostic test for the second and subsequent tests will be reduced by 20%. The second diagnostic test and subsequent tests should be reported with a -51 modifier. Professional services such as gonioscopy, extended ophthalmoscopy and provocative glaucoma testing are excluded from this policy. Visual evoked potential testing is excluded from this policy.
Modifier 51
This modifier is used to identify the secondary procedure or when multiple procedures are performed on the same day by the same provider. List the major primary procedure first and append the modifier to the subsequent procedures. The primary procedure is the one with the highest dollar value.
| Diagnosis Code | Procedure Code | Modifier | Quantity | Payor | Amount Allowed |
| E11.39 - Type 2 diabetes mellitus with other diabetic ophthalmic complication | 92012 - Medical eye examination | 1 | Medicare | 87.69 | |
| E11.3293 - Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy without macular edema, bilateral | 92250 - Fundus photography | 59 | 1 | Medicare | 80.06 |
| E11.3293 - Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy without macular edema, bilateral | 92134 - Retinal laser scan | 51 | 1 | Medicare | 43.28 |
| E11.3293 - Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy without macular edema, bilateral | 92083 - Visual field examination | 51 | 1 | Medicare | 59.63 |
| E11.39 - Type 2 diabetes mellitus with other diabetic ophthalmic complication | 5010F - Communication with managing M.D. | Medicare | 0.00 | ||
| E11.39 - Type 2 diabetes mellitus with other diabetic ophthalmic complication | G8397 - Dilated fundus exam | Medicare | 0.00 | ||
| E11.39 - Type 2 diabetes mellitus with other diabetic ophthalmic complication | 1036F -Patient screened for tobacco use | Medicare | 0.00 | ||
| E11.39 - Type 2 diabetes mellitus with other diabetic ophthalmic complication | G8427 - Current medication documented | Medicare | 0.00 | ||
| Total | $270.66 |
E11.3293
Type 2 diabetes mellitus with mild
nonproliferative diabetic retinopathy
without macular edema, bilateral
362.01
Diabetic retinopathy
92015
Refraction
92020
Gonioscopy
92083
Visual field examination
92250
Fundus photography
92225
Extended ophthalmoscopy
92134
Retinal laser scan
92275
Electroretinography
92283
Color vision examination




 Print | Share
Print | Share