ICD-10 Diagnosis Codes:
H40.231–Intermittent Angle-Closure Glaucoma, right eye
H40.232–Intermittent Angle-Closure Glaucoma, left eye
H40.233–Intermittent Angle-Closure Glaucoma, bilateral
Title
Intermittent Angle-Closure Glaucoma
Category
Glaucoma
Description
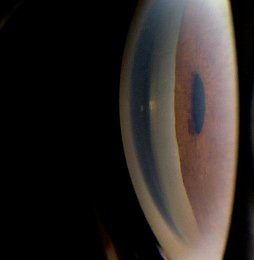
Intermittent angle-closure indicates there is an obstruction to the aqueous outflow mechanism in the anterior chamber.
Glaucoma is an optic neuropathy showing distinctive changes in optic nerve morphology without associated pallor.
The term “glaucoma” refers to a group of chronic, progressive optic neuropathies that have in common characteristic morphologic changes at the optic nerve and retinal nerve fiber layer.
The angle-closure glaucomas are associated with the following clinical features:
- Aqueous outflow restrictions
- Unphysiologic intraocular pressure
- Abnormal ocular perfusion
- Abnormal rate of apoptosis
- Progressive retinal ganglion cell loss
- Characteristic changes in optic nerve anatomy
Retinal Nerve Fiber Layer (RNFL)
- The retinal nerve fiber layer contains all the ganglion cells
- The structure has a striated appearance traversing across the major blood vessels and it can be seen with an ophthalmoscope
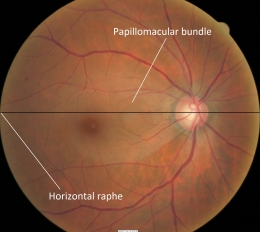
- Loss of vision from glaucoma is the result of axonal damage at the level of the optic disc and retinal ganglion cell axons follow an arcuate path to the optic disc
- Axons from the temporal retina curve around the macular area area, while neurons from the temporal superior and inferior retinal areas stay apart and respect the horizontal raphe
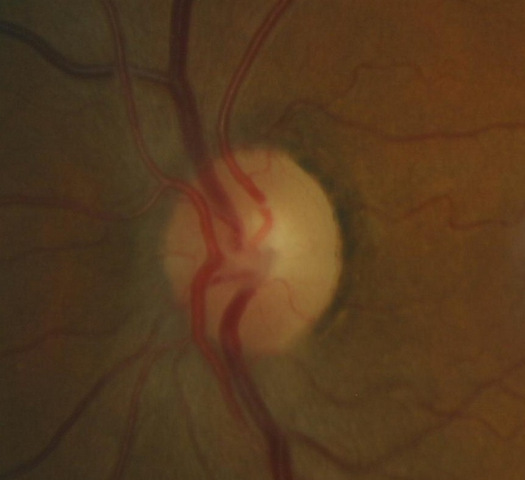 |
Changes in Coloration of the Optic Nerve
|
|
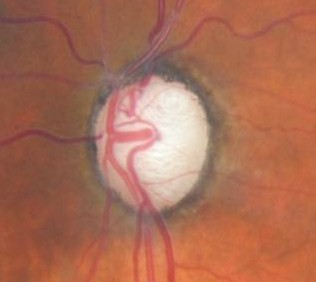 |
Changes in Coloration of the Optic Nerve
|
|
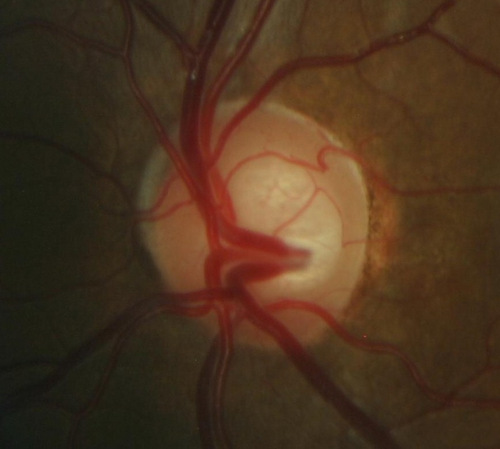 |
Changes in Position of the Optic Cup
|
|
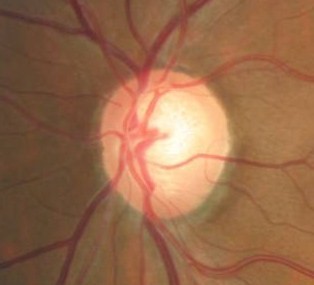 |
Changes in the Margins of the Optic Cup
|
|
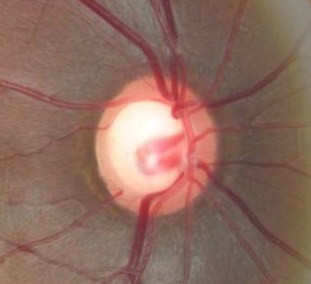 |
Changes in the Optic Cup Depth
|
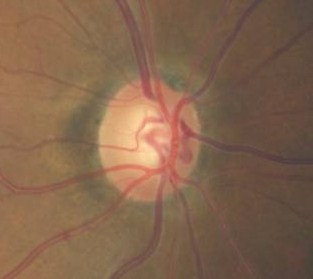 |
Changes in the Optic Size
|
|
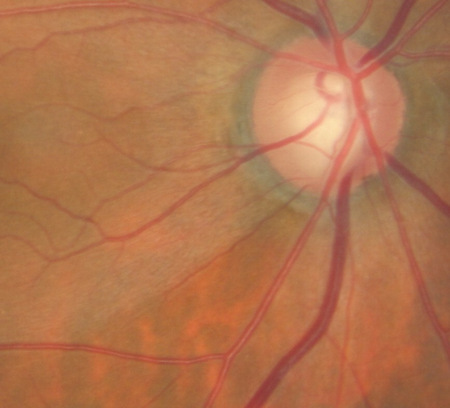 |
Changes in Optic Cup Size
|
|
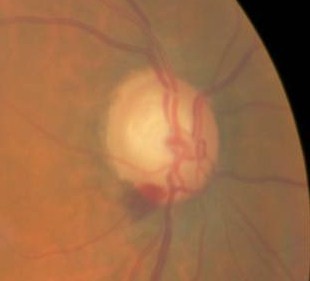 |
Vessel Changes at the Optic Nerve
|
|
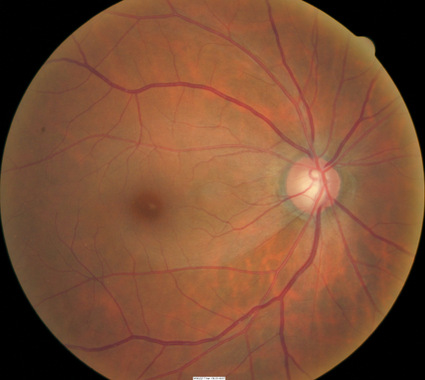 |
Loss of Retinal Ganglion Cells
|
The main goal of the diagnostic evaluation in a patient with glaucoma is to accomplish the following:
1. Determine the presence of glaucoma
2. Classify the type of glaucoma
- High-tension glaucoma
- Normal-tension glaucoma
- Angle-closure glaucoma
- Glaucoma associated with other disorders and/or conditions
3. Assess the amount of glaucomatous damage to the visual system
- Mild damage
- Moderate damage
- Advanced damage
4. Establish a target pressure range
5. Prescribe a treatment program
- Topical medication
- Laser surgery
- Oral medication
- Implant surgery
- Filter surgery
6. Monitor bi-weekly until target achieved
7. Assess optic nerve, intraocular pressure, visual fields, electrodiagnostics, and/or retinal nerve fiber layer in six months
Patient History
Patients will usually be asymptomatic in the early stages of glaucoma. If the patient is complaining of a loss of visual field or visual acuity, the glaucoma is in an advanced stage of its progression.
Intraocular Pressure
Abnormal clinical signs include intraocular pressure in any of the following presentations:
- IOP above 22 mm Hg as measured by applanation tonometry
- Measurements above 24 mm Hg are clinically significant
- 99.85% of the population have IOPs below 24 mm Hg
- Increase in IOP over time
- Asymmetric > 5 mm Hg
- Diurnal variation > 6 mm Hg
Pupillary Examination
- A relative afferent pupillary defect (RAPD) may be present if one eye is more affected than the other
External Ocular Examination with Biomicroscopy
Abnormal clinical signs in the anterior chamber that are associated with glaucoma:
- Corneal endothelial pigment deposits
- Anterior or posterior synechiae
- Other signs of previous intraocular inflammation
- Pseudoexfoliation of the lens
Ophthalmoscopic Examination
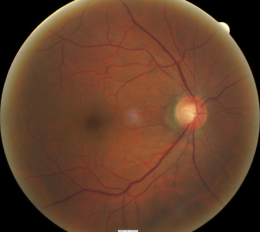
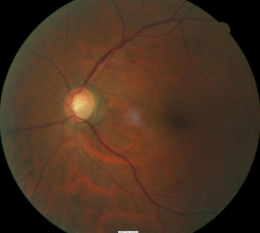
Abnormal clinical signs on the optic disc and the retinal nerve fiber layer (RNFL) that are associated with glaucoma:
1. Changes in the optic nerve
- Coloration
- Appearance of the neuroretinal rim
2. Changes in optic cup
- Shape
- Position
- Margins
- Depth
- Size
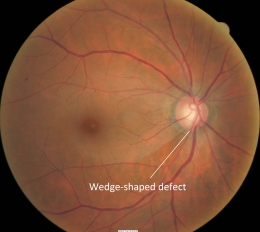
3. Changes in retinal nerve fiber layer
- Wedge-shaped defects
- Diffuse Defects
 |
 |
DIAGNOSTIC TESTS
The following diagnostic tests can provide clincial information in the evaluation of glaucoma and its stage of damage:
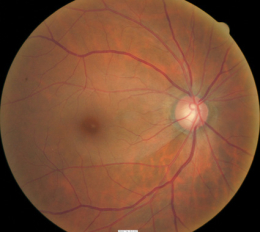
Fundus Photography
- To document the progress or lack of progress of glaucoma
- To document the delivery of medical treatment
- To document the response to treatment
- To help plan a treatment program
- Fundus autofluorescence imaging can be used to detect structural abnormalities and predict functional deficits before standard color fundus photography
- Multi-spectral imaging with the Annidis RHA System can be used to detect structural abnormalities and predict functional deficits before standard color fundus photography
Retinal Scanning Laser
- Can show attenuation of the retinal nerve fiber layer
- Excavation of the optic disc
Visual Field Examination
Research has shown that over 50% of the nerve fibers in any given RNFL bundle can be irreversily damaged before any visual field loss occurs. Depending upon visual fields to separate those patients with glaucoma from those without the disease would still miss a large number of patients.
For most patients with glaucoma, there appears to be about a five year interval between the loss of enough nerve fibers to create a visual field defect during a visual field examination. Relying on visual fields alone to diagnose glaucoma could mean delayed treatment for your glaucoma patients and unnecessary loss of visual field in these people.
Visual field defects will depend on the severity of the stage
- No detectable visual field defect
- Mild reduction in retinal sensitivity
-
Definite glaucomatous visual field defects
- nasal steps
- paracentral scotomas
- arcuate scotomas
- Temporal changes in field patterns
Gonioscopy
- Can show abnormal angle structures
- Abnormal pigmentation of the trabecular meshwork
- Abnormal anterior chamber anatomy
- Abnormal iris configuration
Corneal Pachymetry
- A central corneal thickness of less than 500 microns is a risk factor for glaucoma
Extended Ophthalmoscopy
- Evaluate optic disc morphology
- Document structural changes to the optic disc
Electrodiagnostics
- Visual evoked potential testing evaluates the function of the afferent visual sensory system
- Electroretinography evaluates the function of the retinal ganglion cells
Serial Tonometry
- Establish diurnal curve
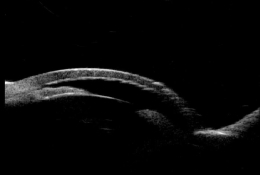
Ultrasound Biomicroscopy (UBM) and/or Anterior Segment Imaging
- Determine iris configuration
- Evaluate anterior chamber anatomy
Refraction
- Measurement of visual acuity
- Measurement of visual function
Provocative Tests for Glaucoma
- Risk assessment for angle-closure glaucomas
Glaucoma may be congenital or acquired and it can be further sub-classified into open-angle or angle-closure types based on anterior chamber anatomy. Most patients have one of the acquired forms of the disease, and the open-angle glaucomas are the most common type.
Narrow-angle glaucomas can be sub-classified into normal tension or elevated tension types based on intraocular pressure. Elevated tension glaucomas can be further sub-classified into primary or secondary types based on the presence or absence of associated factors contributing to the elevated intraocular pressure.
Differential diagnosis will depend on if the damage is observed on the optic nerve or a visual field defect is present. Below is a list of possible differential diagnosis:
Optic Nerve Head
- Congenital and hereditary anomalies of the disc
- Ischemic optic neuropathy
- Compressive lesions of the optic nerve
Visual Field Defect
- Pituitary tumors
- Ischemic optic neuropathy
- Vascular disease
- Drusen of the optic nerve
- Neurological condition
When initiating treatment, assume that the pre-treatment measurement of intraocular pressure (IOP) is the level that produced damage to the optic nerve. If the IOP remained at this level, additional damage to the optic nerve would follow.
To treat glaucoma, an IOP level is identified below which further optic nerve damage is unlikely to occur. This IOP level is the target pressure range and it is selected based on the following:
- Pre-treatment level of IOP
- The rapidity with which the damage to the optic nerve occurred, if that is known
- Patient age
- General health of the patient
Treatment should maintain the IOP at or below the target level. The status of the optic nerve, visual fields, electrodiagnostics, and the retinal nerve fiber layer are monitored over time for evidence of stability or deterioration. In the event of further damage, the target pressure range is reset to a lower level.
Pharmacologic Treatment
Select your initial medication based upon target pressure range, medical history, and ocular history. If the initial medication fails to achieve the target pressure range within one month – discontinue the initial medication and substitute another.
If the second medication fails to achieve the target pressure, begin combination therapy by using two different medications. If this strategy fails, you could add still a third class of glaucoma medications to the regimen. With three different eye drops, non-compliance becomes common and laser surgery may be a better treatment option.
Prostaglandins are the most popular class of medications for glaucoma therapy due to their excellent efficacy, safety index and tolerability. They flatten the diurnal curve significantly and achieve the greatest IOP reduction of any class of topical medication.
Classes of Glaucoma Medications
Prostaglandins
- Lumigan
- Travatan Z
- Zioptan
- Xalatan
Beta-Blockers
- Timolol
- Betagan
- Betimol
- OptiPranolol
Adrenergic Agonists
- Alphagan P 0.1% and 0.15%
- brimonidine 0.2%
- Propine
Carbonic Anhydrase Inhibitors
- Azopt
- Trusopt
Cholinergic Agonists
- Pilocarpine
- Carbachol
- Echothiophate
Docosanoids
- Rescula
Combination Glaucoma Medications
- Cosopt – carbonic anhydrase inhibitor / beta-blocker
- Cosopt PF – carbonic anhydrase inhibitor / beta-blocker
- Combigan – adrenergic agonist / beta-blocker
- Simbrinza – adrenergic agonist / carbonic anhydrase inhibitor
Changing the Treatment due to Side Effects
Prostaglandins
- Red eye
- Eye color change
- Excessive eyelash growth
- Uveitis
- Macular edema
Beta-Blockers
- Bronchospasm
- Pulmonary edema
- Heart failure
- Headache
- Weakness
- Depression
- Lethargy
- Itching
- Stinging
- Blurred vision
- Photophobia
- Loss of libido
Adrenergic Agonist
- Red eye
- Allergic follicular conjunctivitis
- Dry mouth
- Drowsiness
Carbonic Andydrase Inhibitors
- Sulfa allergy
- Red eye
- Ocular itching
- Metallic taste
- Paresthesia of fingers or toes
- Headache
- Blood dyscrasias
- Depression
- Loss of libido
- Impotence
Cholinergic Agonists
- Red eye
- Induced myopia
- Reduced vision in low illumination
- Headaches
- Lens opacities
- Iris cysts
- Increased risk of angle closure
- Increased risk of retinal detachment
- Sweating
- Salivation
- Diarrhea
- Bradycardia
- Dyspnea
Docosanoids
- Eye color change
- Stinging
- Increase in periocular eyelid pigmentation
- Increase in upper eyelid sulcus deepening
1. Dada T, Kumar G, Mishra SJ. Ultrasound Biomicroscopy in Glaucoma: An Update. Journal of Current Glaucoma Practice. January 2008.
2. Al-Aswad L, Tsai J. Managing the Narrow-Angle Patient. RevOphth. 30 June 2004. http://www.revophth.com/content/i/1330/c/25527/. Last accessed May 30, 2014.
3. Ventura LM, Golubev I, Feuer W, Porciatti V. The PERG in Diabetic Glaucoma Suspects With No Evidence of Retinopathy. J Glaucoma 2010; 19(4): 243-247.
4. Tsai JC. VEP Technology for the Detection of Glaucomatous Visual Field Loss. Glaucoma Today. 2009 March. http://glaucomatoday.com/2009/03/GT0309_10.php/. Last accessed June 25, 2015.
5. Banitt M, Ventura L, Feuer W, Savatovsky E, Luna G, Shif O, Bosse B, Porciatti V. Progressive Loss of Retinal Ganglion Cell Function Precedes Structural Loss by Several Years in Glaucoma Suspects. National Institutes of Health-National Eye Institute. 7 Feb 2013. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3626526/. Last accessed June 25, 2015.
6. Malik R, Swanson W, Garway-Teath D. The ‘Structure-function’ Relationship in Glaucoma — Past Thinking and Current Concepts. Clin Experiment Ophthalmol. 2012 May-Jun;40(4):369-80. doi: 10.1111/j.1442-9071.2012.02770.x. Epub 2012 Apr 12. http://www.ncbi.nlm.nih.gov/pubmed/22339936. Last accessed June 29, 2015.
7. Bremmer F.D. Pupil Assessment in Optic Nerve Disorders. Eye (Lond). 2004 Nov;18(11):1175-81. http://www.ncbi.nlm.nih.gov/pubmed/15534603. Last accessed June 29, 2015.
8. Chew S, Cunningham W, Gamble G, Danesh-Meyer H. Retinal Nerve Fiber Layer Loss in Glaucoma Patients with a Relative Afferent Pupillary Defect. Invest Ophthalmol Vis Sci. 2010 Oct;51(10):5049-53. doi: 10.1167/iovs.09-4216. Epub 2010 May 5. http://www.ncbi.nlm.nih.gov/pubmed/20445112. Last accessed June 29, 2015.
9. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial Variations in the Prevalence of Primary Open-Angle Glaucoma The Baltimore Eye Survey. JAMA. 1991 Jul 17;266(3):369-74. http://www.ncbi.nlm.nih.gov/pubmed/2056646. Last accessed June 29, 2015.
10. Rabin J, Gooch J, Ivan D. Rapid Quantification of Color Vision: The Cone Contrast Test. Invest Ophthalmol Vis Sci. 2011 Feb 9;52(2):816-20. doi: 10.1167/iovs.10-6283. http://www.ncbi.nlm.nih.gov/pubmed/21051721. Last accessed June 29, 2015.
365.21
Intermittent angle-closure glaucoma
92133
Retinal nerve fiber layer scan
92083
Visual field examination
92250
Fundus photography
92225
Extended ophthalmoscopy
92226
Subsequent ophthalmoscopy
92020
Gonioscopy
95930
Visual evoked potential
92275
Electroretinography
92100
Serial tonometry
92132
Anterior segment imaging
76513
Anterior segment ultrasound
76514
Corneal pachymetry
Occurrence
The prevalence of the incidence of narrow-angle glaucoma is less than 1% of the general population.
Distribution
Glaucoma is not distributed evenly throughout the population.
- More women than men develop glaucoma
- Inuit Eskimos and East Asians
Risk Factors
- Vertically elongated cup-to-disc ratios
- Asymmetric cup-to-disc ratios
- Intraocular pressures greater than 21 mm Hg
- Advancing age
- Increasing crystalline lens thickness
- Hyperopia




 Print | Share
Print | Share